Stereoelectronic Tuning of Bioinspired Nonheme Iron(IV)-Oxo Species by Amide Groups in Primary and Secondary Coordination Spheres for Selective Oxygenation Reactions
IF 4.3
2区 化学
Q1 CHEMISTRY, INORGANIC & NUCLEAR
引用次数: 0
Abstract
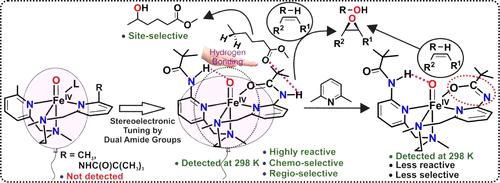
Two mononuclear iron(II) complexes, [(6-amide2-BPMEN)FeII](OTf)2 (1) and [(6-amide-Me-BPMEN)FeII(OTf)](OTf) (2), supported by two BPMEN-derived (BPMEN = N1,N2-dimethyl-N1,N2-bis(pyridine-2-yl-methyl)ethane-1,2-diamine) ligands bearing one or two amide functionalities have been isolated to study their reactivity in the oxygenation of C–H and C═C bonds using isopropyl 2-iodoxybenzoate (iPr-IBX ester) as the oxidant. Both 1 and 2 contain six-coordinate high-spin iron(II) centers in the solid state and in solution. The 6-amide2-BPMEN ligand stabilizes an S = 1 iron(IV)-oxo intermediate, [(6-amide2-BPMEN)FeIV(O)]2+ (1A). The oxidant (1A) oxygenates the C–H and C═C bonds with a high selectivity. Oxidant 1A, upon treatment with 2,6-lutidine, is transformed into another oxidant [{(6-amide2-BPMEN)-(H)}FeIV(O)]+ (1B) through deprotonation of an amide group, resulting in a stronger equatorial ligand field and subsequent stabilization of the triplet ground state. In contrast, no iron-oxo species could be observed from complex 2 and [(6-Me2-BPMEN)FeII(OTf)2] (3) under similar experimental conditions. The iron(IV)-oxo oxidant 1A shows the highest A/K selectivity in cyclohexane oxidation and 3°/2° selectivity in adamantane oxidation reported for any synthetic nonheme iron(IV)-oxo complexes. Theoretical investigation reveals that the hydrogen bonding interaction between the −NH group of the noncoordinating amide group and Fe═O core smears out the equatorial charge density, reducing the triplet–quintet splitting, and thus helping complex 1A to achieve better reactivity.
通过一级和二级配位层中的酰胺基团对生物启发的非血红素铁(IV)-氧化物进行立体电子调谐,以实现选择性氧合反应
两个单核铁(II)配合物,[(6-amid2-BPMEN)FeII](OTf)2 (1) 和 [(6-amid-Me-BPMEN)FeII(OTf)](OTf)(2),由两个 BPMEN 衍生(BPMEN = N1,N2-二甲基-N1,N2-双(吡啶-2-基-甲基)乙烷-1、2-iodoxybenzoate 异丙酯(iPr-IBX 酯)作为氧化剂,研究它们在 C-H 键和 C═C 键氧合反应中的反应活性。1 和 2 在固态和溶液中都含有六配位高自旋铁(II)中心。6-amide2-BPMEN 配体能稳定 S = 1 的铁(IV)-氧代中间体 [(6-amide2-BPMEN)FeIV(O)]2+ (1A)。氧化剂 (1A) 以高选择性使 C-H 键和 C═C 键含氧。氧化剂 1A 经 2,6 - 丁烷处理后,会通过酰胺基团的去质子化作用转化为另一种氧化剂 [{(6-amide2-BPMEN)-(H)}FeIV(O)]+ (1B),从而产生更强的赤道配位体场,进而稳定三重基态。相反,在类似的实验条件下,复合物 2 和 [(6-Me2-BPMEN)FeII(OTf)2] (3) 中没有观察到铁氧物种。铁(IV)-氧氧化剂 1A 在环己烷氧化中显示出最高的 A/K 选择性,在金刚烷氧化中显示出 3°/2°的选择性。理论研究表明,非配位酰胺基团的 -NH 基团与 Fe═O 核心之间的氢键相互作用抹去了赤道电荷密度,降低了三重-五重分裂,从而帮助复合物 1A 获得更好的反应活性。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Inorganic Chemistry
化学-无机化学与核化学
CiteScore
7.60
自引率
13.00%
发文量
1960
审稿时长
1.9 months
期刊介绍:
Inorganic Chemistry publishes fundamental studies in all phases of inorganic chemistry. Coverage includes experimental and theoretical reports on quantitative studies of structure and thermodynamics, kinetics, mechanisms of inorganic reactions, bioinorganic chemistry, and relevant aspects of organometallic chemistry, solid-state phenomena, and chemical bonding theory. Emphasis is placed on the synthesis, structure, thermodynamics, reactivity, spectroscopy, and bonding properties of significant new and known compounds.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


