Improving the Electrocatalytic Activity of a Core/Shell NiCo–ZIF@PBA Catalyst by Co–O–Fe Bridge Bonds for Water Oxidation
IF 4.3
2区 化学
Q1 CHEMISTRY, INORGANIC & NUCLEAR
引用次数: 0
Abstract
In response to the limitations of slow reaction kinetics and elevated overpotentials in the OER, a novel core–shell structured electrocatalyst, termed NiCo–ZIF-67@Fe–Co–Ni-PBA or NiCo–ZIF@PBA, has been developed. This material demonstrates exceptional catalytic performance, exhibiting a minimal overpotential of approximately 188 mV at 10 mA cm–2, alongside a Tafel slope of 109 mV dec–1. Its robust stability in a 1 M KOH solution during the OER operations is noteworthy. Theoretical insights from DFT calculations reveal that a Co–O–Fe bridging configuration within NiCo–ZIF@PBA lowers the energy barrier for the reaction to 1.79 eV, a significant reduction from 2.67 eV observed with NiCo–ZIF-67. The improvement in electrochemical performance is primarily due to the emergence of Co3+ ions, which results from the efficient charge transfer occurring at the interface of the PBA and NiCo–ZIF core–shell structure. These findings suggest a promising strategy for designing advanced core–shell materials for electrocatalytic applications.
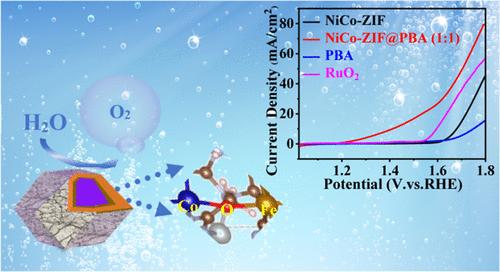
通过 Co-O-Fe 桥键提高核/壳 NiCo-ZIF@PBA 催化剂在水氧化中的电催化活性
针对 OER 中反应动力学缓慢和过电位升高的限制,我们开发了一种新型核壳结构电催化剂,称为 NiCo-ZIF-67@Fe-Co-Ni-PBA,或 NiCo-ZIF@PBA。这种材料具有卓越的催化性能,在 10 mA cm-2 的条件下,过电位最低约为 188 mV,塔菲尔斜率为 109 mV dec-1。值得注意的是,在 OER 操作过程中,该材料在 1 M KOH 溶液中具有很强的稳定性。DFT 计算的理论见解表明,NiCo-ZIF@PBA 中的 Co-O-Fe 桥接构型将反应能垒降低到了 1.79 eV,比在 NiCo-ZIF-67 中观察到的 2.67 eV 明显降低。电化学性能的改善主要归功于 Co3+ 离子的出现,这是在 PBA 和 NiCo-ZIF 核壳结构界面上发生的高效电荷转移的结果。这些发现为设计用于电催化应用的先进核壳材料提供了一种前景广阔的策略。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Inorganic Chemistry
化学-无机化学与核化学
CiteScore
7.60
自引率
13.00%
发文量
1960
审稿时长
1.9 months
期刊介绍:
Inorganic Chemistry publishes fundamental studies in all phases of inorganic chemistry. Coverage includes experimental and theoretical reports on quantitative studies of structure and thermodynamics, kinetics, mechanisms of inorganic reactions, bioinorganic chemistry, and relevant aspects of organometallic chemistry, solid-state phenomena, and chemical bonding theory. Emphasis is placed on the synthesis, structure, thermodynamics, reactivity, spectroscopy, and bonding properties of significant new and known compounds.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


