Home-based transcranial direct current stimulation treatment for major depressive disorder: a fully remote phase 2 randomized sham-controlled trial
IF 50
1区 医学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
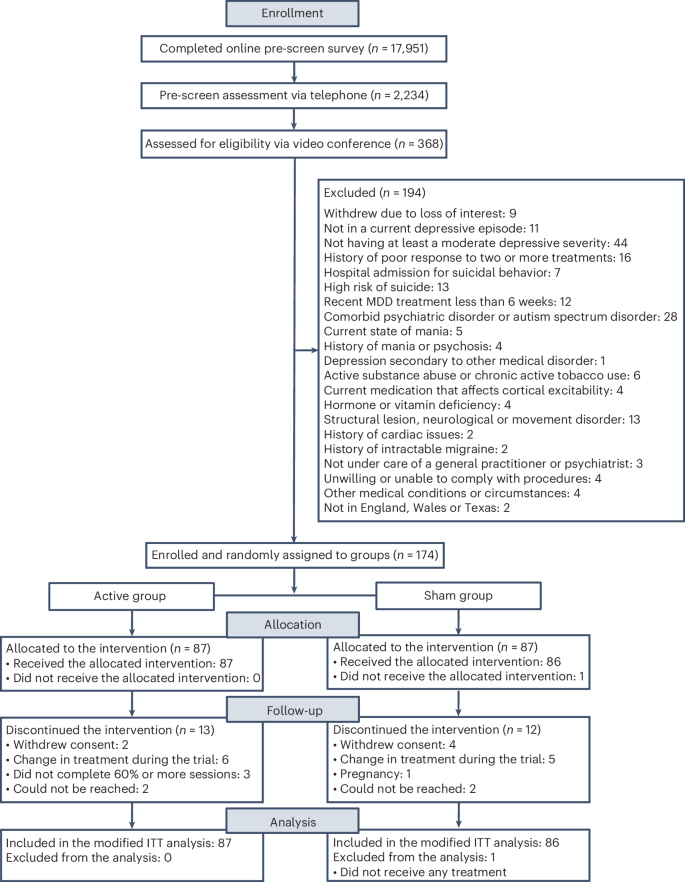
Transcranial direct current stimulation (tDCS) has been proposed as a new treatment in major depressive disorder (MDD). This is a fully remote, multisite, double-blind, placebo-controlled, randomized superiority trial of 10-week home-based tDCS in MDD. Participants were 18 years or older, with MDD in current depressive episode of at least moderate severity as measured using the Hamilton Depression Rating Scale (mean = 19.07 ± 2.73). A total of 174 participants (120 women, 54 men) were randomized to active (n = 87, mean age = 37.09 ± 11.14 years) or sham (n = 87, mean age = 38.32 ± 10.92 years) treatment. tDCS consisted of five sessions per week for 3 weeks then three sessions per week for 7 weeks in a 10-week trial, followed by a 10-week open-label phase. Each session lasted 30 min; the anode was placed over the left dorsolateral prefrontal cortex and the cathode over the right dorsolateral prefrontal cortex (active tDCS 2 mA and sham tDCS 0 mA, with brief ramp up and down to mimic active stimulation). As the primary outcome, depressive symptoms showed significant improvement when measured using the Hamilton Depression Rating Scale: active 9.41 ± 6.25 point improvement (10-week mean = 9.58 ± 6.02) and sham 7.14 ± 6.10 point improvement (10-week mean = 11.66 ± 5.96) (95% confidence interval = 0.51–4.01, P = 0.012). There were no differences in discontinuation rates. In summary, a 10-week home-based tDCS treatment with remote supervision in MDD showed high efficacy, acceptability and safety. ClinicalTrials.gov registration: NCT05202119 A randomized, sham-controlled, superiority trial of a 10-week course of home-based transcranial direct current stimulation found greater improvements in depressive symptoms with active compared to sham stimulation in major depressive disorder.

基于家庭的经颅直流电刺激治疗重度抑郁障碍:完全远程 2 期随机假对照试验
经颅直流电刺激(tDCS)被认为是治疗重度抑郁症(MDD)的一种新疗法。这是一项完全远程、多站点、双盲、安慰剂对照、随机优越性试验,对重度抑郁症患者进行为期 10 周的家庭经颅直流电刺激治疗。参与者年龄在 18 岁或以上,患有 MDD,当前抑郁发作程度至少为中度,以汉密尔顿抑郁量表(Hamilton Depression Rating Scale)测量(平均值 = 19.07 ± 2.73)。共有 174 名参与者(120 名女性,54 名男性)被随机分配到积极治疗(87 人,平均年龄为 37.09 ± 11.14 岁)或假治疗(87 人,平均年龄为 38.32 ± 10.92 岁)中。每次治疗持续 30 分钟;阳极置于左侧背外侧前额叶皮层,阴极置于右侧背外侧前额叶皮层(活性 tDCS 2 毫安,假性 tDCS 0 毫安,通过短暂的上下斜坡来模拟活性刺激)。作为主要研究结果,使用汉密尔顿抑郁量表(Hamilton Depression Rating Scale)测量的抑郁症状有显著改善:主动改善 9.41 ± 6.25 分(10 周平均 = 9.58 ± 6.02),假改善 7.14 ± 6.10 分(10 周平均 = 11.66 ± 5.96)(95% 置信区间 = 0.51-4.01,P = 0.012)。停药率没有差异。总之,在远程监护下对 MDD 进行为期 10 周的居家 tDCS 治疗具有很高的疗效、可接受性和安全性。ClinicalTrials.gov 注册:NCT05202119
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature Medicine
医学-生化与分子生物学
CiteScore
100.90
自引率
0.70%
发文量
525
审稿时长
1 months
期刊介绍:
Nature Medicine is a monthly journal publishing original peer-reviewed research in all areas of medicine. The publication focuses on originality, timeliness, interdisciplinary interest, and the impact on improving human health. In addition to research articles, Nature Medicine also publishes commissioned content such as News, Reviews, and Perspectives. This content aims to provide context for the latest advances in translational and clinical research, reaching a wide audience of M.D. and Ph.D. readers. All editorial decisions for the journal are made by a team of full-time professional editors.
Nature Medicine consider all types of clinical research, including:
-Case-reports and small case series
-Clinical trials, whether phase 1, 2, 3 or 4
-Observational studies
-Meta-analyses
-Biomarker studies
-Public and global health studies
Nature Medicine is also committed to facilitating communication between translational and clinical researchers. As such, we consider “hybrid” studies with preclinical and translational findings reported alongside data from clinical studies.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


