TME-responsive nanoplatform for multimodal imaging-guided synergistic precision therapy of esophageal cancer via inhibiting HIF-1α signal pathway
IF 10.5
1区 医学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
Esophageal cancer (EC) is the sixth leading cause of cancer-related deaths, and its treatment poses significant challenges. In recent years, photodynamic, photothermal, and chemodynamic therapies have emerged as alternative strategies for tumor intervention. However, limitations such as poor tumor targeting, insufficient microenvironment responsiveness, and unclear mechanisms hinder their application. In this study, we found that hypoxia-inducible factor 1 alpha (HIF-1α) was highly expressed in clinical EC samples, which contributed to tumor malignancy and metastasis. We developed a carbon dots (CDs)-based tumor microenvironment (TME)-responsive nanoplatform, CDs-MnO2-Au-Cet (CMAC), designed for multimodal imaging-guided precision therapy in EC. Both in vitro and in vivo experiments demonstrated that CMAC effectively targeted and imaged EC cells and tissues. CMAC significantly inhibited tumor growth by inducing apoptosis and reducing lung metastasis. Mechanistically, CMAC administration led to a substantial downregulation of HIF-1α and its downstream targets, GLUT1 and MMP9. In summary, we presented a novel nanoplatform for imaging-guided synergistic therapy in EC, which demonstrated excellent anti-tumor growth and metastasis capabilities, along with favorable biocompatibility. This study laid the groundwork for developing innovative theranostic strategies for EC.
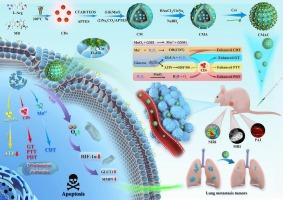
TME响应纳米平台通过抑制HIF-1α信号通路,在多模态成像引导下协同精准治疗食管癌。
食管癌(EC)是导致癌症相关死亡的第六大原因,其治疗面临着巨大挑战。近年来,光动力疗法、光热疗法和化学动力疗法已成为干预肿瘤的替代策略。然而,肿瘤靶向性差、微环境反应性不足、机制不清等局限性阻碍了它们的应用。在这项研究中,我们发现缺氧诱导因子1α(HIF-1α)在临床EC样本中高表达,而缺氧诱导因子1α是导致肿瘤恶变和转移的原因之一。我们开发了一种基于碳点(CDs)的肿瘤微环境(TME)响应纳米平台--CDs-MnO2-Au-Cet(CMAC),设计用于EC的多模态成像引导精准治疗。体外和体内实验均证明,CMAC 能有效靶向和成像EC细胞和组织。CMAC 通过诱导细胞凋亡和减少肺转移,明显抑制了肿瘤的生长。从机理上讲,CMAC 能大幅下调 HIF-1α 及其下游靶标 GLUT1 和 MMP9。总之,我们提出了一种用于EC成像引导协同治疗的新型纳米平台,该平台具有出色的抗肿瘤生长和转移能力,以及良好的生物相容性。这项研究为开发针对EC的创新治疗策略奠定了基础。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Controlled Release
医学-化学综合
CiteScore
18.50
自引率
5.60%
发文量
700
审稿时长
39 days
期刊介绍:
The Journal of Controlled Release (JCR) proudly serves as the Official Journal of the Controlled Release Society and the Japan Society of Drug Delivery System.
Dedicated to the broad field of delivery science and technology, JCR publishes high-quality research articles covering drug delivery systems and all facets of formulations. This includes the physicochemical and biological properties of drugs, design and characterization of dosage forms, release mechanisms, in vivo testing, and formulation research and development across pharmaceutical, diagnostic, agricultural, environmental, cosmetic, and food industries.
Priority is given to manuscripts that contribute to the fundamental understanding of principles or demonstrate the advantages of novel technologies in terms of safety and efficacy over current clinical standards. JCR strives to be a leading platform for advancements in delivery science and technology.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


