Steviol rebaudiosides bind to four different sites of the human sweet taste receptor (T1R2/T1R3) complex explaining confusing experiments
IF 5.9
2区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
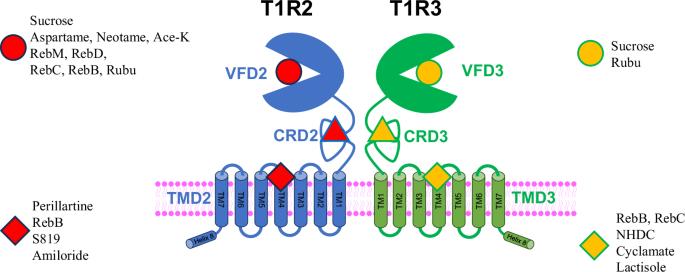
Sucrose provides both sweetness and energy by binding to both Venus flytrap domains (VFD) of the heterodimeric sweet taste receptor (T1R2/T1R3). In contrast, non-caloric sweeteners such as sucralose and aspartame only bind to one specific domain (VFD2) of T1R2, resulting in high-intensity sweetness. In this study, we investigate the binding mechanism of various steviol glycosides, artificial sweeteners, and a negative allosteric modulator (lactisole) at four distinct binding sites: VFD2, VFD3, transmembrane domain 2 (TMD2), and TMD3 through binding experiments and computational docking studies. Our docking results reveal multiple binding sites for the tested ligands, including the radiolabeled ligands. Our experimental evidence demonstrates that the C20 carboxy terminus of the Gα protein can bind to the intracellular region of either TMD2 or TMD3, altering GPCR affinity to the high-affinity state for steviol glycosides. These findings provide a mechanistic understanding of the structure and function of this heterodimeric sweet taste receptor. Sucrose and other non-caloric sweeteners can bind to different domains of the heterodimeric sweet taste receptor (T1R2/T1R3), resulting in different levels of sweetness. Here, the authors investigate the binding mechanism of various steviol glycosides, artificial sweeteners, and a negative allosteric modulator (lactisole) at four distinct binding sites of T1R2/T1R3 through binding experiments and computational docking studies, revealing multiple binding sites for the tested ligands and structural– function correlations of ligand–receptor interactions.
甜菊醇甜菊糖甙与人类甜味受体(T1R2/T1R3)复合物的四个不同位点结合的混乱实验解释
蔗糖通过与异源二聚体甜味受体(T1R2/T1R3)的两个金星捕蝇草结构域(VFD)结合,提供甜味和能量。相比之下,三氯蔗糖和阿斯巴甜等非热量甜味剂只与 T1R2 的一个特定结构域(VFD2)结合,从而产生高强度的甜味。在本研究中,我们研究了各种甜菊醇苷、人工甜味剂和负异位调节剂(乳糖醇)在四个不同结合位点的结合机制:通过结合实验和计算对接研究,我们探究了各种甜菊糖苷、人工甜味剂和负异位调节剂(乳糖醇)在四个不同结合位点的结合机制:VFD2、VFD3、跨膜结构域 2 (TMD2) 和 TMD3。我们的对接结果显示了测试配体(包括放射性标记配体)的多个结合位点。我们的实验证据表明,Gα 蛋白的 C20 羧基末端可与 TMD2 或 TMD3 的细胞内区域结合,从而改变 GPCR 对甜菊醇苷的亲和力,使其处于高亲和力状态。这些发现提供了对这种异源二聚体甜味受体的结构和功能的机理认识。蔗糖和其他非热量甜味剂可以与异源二聚体甜味受体(T1R2/T1R3)的不同结构域结合,从而产生不同程度的甜味。在此,作者通过结合实验和计算对接研究,探讨了各种甜菊糖苷、人工甜味剂和一种负异位调节剂(乳糖醇)在 T1R2/T1R3 四个不同结合位点的结合机制,揭示了所测试配体的多个结合位点以及配体与受体相互作用的结构-功能相关性。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Communications Chemistry
Chemistry-General Chemistry
CiteScore
7.70
自引率
1.70%
发文量
146
审稿时长
13 weeks
期刊介绍:
Communications Chemistry is an open access journal from Nature Research publishing high-quality research, reviews and commentary in all areas of the chemical sciences. Research papers published by the journal represent significant advances bringing new chemical insight to a specialized area of research. We also aim to provide a community forum for issues of importance to all chemists, regardless of sub-discipline.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


