Covalent Inhibitors of S100A4 Block the Formation of a Pro-Metastasis Non-Muscle Myosin 2A Complex
IF 6.8
1区 医学
Q1 CHEMISTRY, MEDICINAL
引用次数: 0
Abstract
The S100 protein family functions as protein–protein interaction adaptors regulated by Ca2+ binding. Formation of various S100 complexes plays a central role in cell functions, from calcium homeostasis to cell signaling, and is implicated in cell growth, migration, and tumorigenesis. We established a suite of biochemical and cellular assays for small molecule screening based on known S100 protein–protein interactions. From 25 human S100 proteins, we focused our attention on S100A4 because of its well-established role in cancer progression and metastasizes by interacting with nonmuscle myosin II (NMII). We identified several potent and selective inhibitors of this interaction and established the covalent nature of binding, confirmed by mass spectrometry and crystal structures. 5b showed on-target activity in cells and inhibition of cancer cell migration. The identified S100A4 inhibitors can serve as a basis for the discovery of new cancer drugs operating via a novel mode of action.
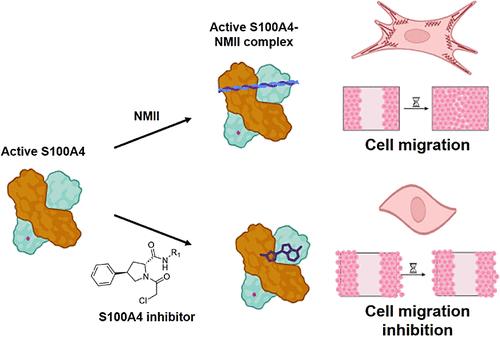
S100A4的共价抑制剂能阻止形成促进转移的非肌球蛋白2A复合物
S100 蛋白家族作为蛋白-蛋白相互作用适配体,受 Ca2+ 结合调节。各种 S100 复合物的形成在细胞功能(从钙平衡到细胞信号传导)中发挥着核心作用,并与细胞生长、迁移和肿瘤发生有关。我们根据已知的 S100 蛋白-蛋白相互作用建立了一套生化和细胞检测方法,用于筛选小分子化合物。从 25 个人类 S100 蛋白中,我们将注意力集中在 S100A4 上,因为它在癌症进展中的作用已得到证实,并通过与非肌球蛋白 II(NMII)相互作用而转移。我们发现了几种抑制这种相互作用的强效选择性抑制剂,并通过质谱分析和晶体结构证实了这种结合的共价性质。5b 在细胞中显示出靶向活性并抑制癌细胞迁移。已发现的 S100A4 抑制剂可作为发现通过新作用模式发挥作用的新型抗癌药物的基础。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Medicinal Chemistry
医学-医药化学
CiteScore
4.00
自引率
11.00%
发文量
804
审稿时长
1.9 months
期刊介绍:
The Journal of Medicinal Chemistry is a prestigious biweekly peer-reviewed publication that focuses on the multifaceted field of medicinal chemistry. Since its inception in 1959 as the Journal of Medicinal and Pharmaceutical Chemistry, it has evolved to become a cornerstone in the dissemination of research findings related to the design, synthesis, and development of therapeutic agents.
The Journal of Medicinal Chemistry is recognized for its significant impact in the scientific community, as evidenced by its 2022 impact factor of 7.3. This metric reflects the journal's influence and the importance of its content in shaping the future of drug discovery and development. The journal serves as a vital resource for chemists, pharmacologists, and other researchers interested in the molecular mechanisms of drug action and the optimization of therapeutic compounds.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


