Synthesis of a novel β-cyclodextrin chiral stationary phase and its application to the evaluation of the enantioselective bioaccumulation and elimination behavior of tebuconazole in Rana nigromaculata tadpoles
IF 5.7
2区 化学
Q1 CHEMISTRY, ANALYTICAL
引用次数: 0
Abstract
Background
The increased production and use of chiral pesticides will enhance their exposure in the environment. Chiral pesticides typically exhibit varied biological effects among these enantiomers. Therefore, it is very essential to develop and validate chiral analytical methods to investigate their potential ecological risks from a stereoselective perspective. Current separation of pesticides enantiomers relies extensively on chiral stationary phases (CSPs), while the development of β-Cyclodextrin derivatives CSPs become the research focus due to their great modifiability and excellent chiral recognition capabilities.
Results
A novel chiral stationary phase, 3,5-dichlorophenylaminomethyl-6-phenylenediamine-β-cyclodextrin chemically bonded silica gel (MPDCDA), was successfully prepared. Based on that, a stereoselective HPLC-MS/MS method was developed and validated for the determination of tebuconazole enantiomers in Rana nigromaculata tadpoles. After extraction by QuEChERS, the tebuconazole enantiomers were completely separated with the resolutions of 1.63 using the mobile phase of methanol-water (70/30, v/v). Good linearity (r > 0.9990) for both enantiomers over a concentration range of 0.20–500.0 ng/mL was obtained with the accuracy ranged from 6.7 % to 9.3 % and the intra-day and inter-day precisions below 6.2 % at three quality control levels. The proposed method was successfully applied in evaluating the enantioselective bioaccumulation and elimination profiles of tebuconazole in tadpoles. At the tested conditions, there was no significantly enantioselective difference in the bioaccumulation process for S- tebuconazole and R-tebuconazole. However, the elimination process of tebuconazole enantiomers was enantioselective with R-tebuconazole preferentially degraded.
Significance
This work provided an accurate risk assessment of chiral pesticides to non-target aquatic organisms from a stereoselective perspective. These findings would deepen our understanding of the potential ecological risks of chiral pesticides on aquatic organisms and provide scientific support for the protection of aquatic organisms and their ecological environments, as well as sustainable development.
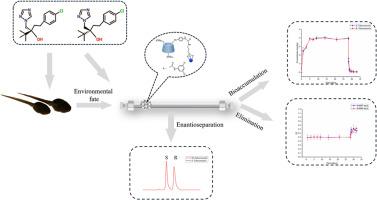
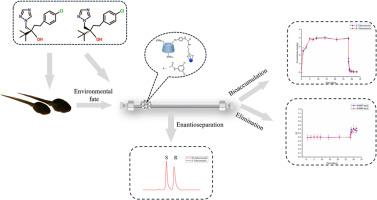
新型 β-环糊精手性固定相的合成及其在评估戊唑醇在黑鳃蛙蝌蚪中的对映体选择性生物累积和消除行为中的应用
背景手性农药生产和使用的增加会增加其在环境中的暴露量。手性农药的对映体通常具有不同的生物效应。因此,开发和验证手性分析方法以从立体选择性角度研究其潜在的生态风险非常重要。目前农药对映体的分离广泛依赖于手性固定相(CSPs),而β-环糊精衍生物 CSPs 因其极佳的可调控性和优异的手性识别能力而成为研究的重点。在此基础上,建立并验证了一种立体选择性 HPLC-MS/MS 方法,用于测定黑线蛙蝌蚪中戊唑醇对映体的含量。经QuEChERS萃取后,以甲醇-水(70/30,v/v)为流动相,戊唑醇对映体完全分离,分辨率为1.63。在0.20-500.0 ng/mL浓度范围内,两种对映体的线性关系良好(r > 0.9990),在3个质控水平下,准确度为6.7%-9.3%,日内和日间精密度低于6.2%。该方法成功地应用于评价戊唑醇在蝌蚪体内的对映体选择性生物累积和消除谱图。在测试条件下,S-戊唑醇和 R-戊唑醇的生物累积过程没有明显的对映体选择性差异。不过,戊唑醇对映体的消除过程具有对映选择性,R- 戊唑醇优先降解。这些发现将加深我们对手性农药对水生生物潜在生态风险的认识,为水生生物及其生态环境的保护和可持续发展提供科学支持。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Analytica Chimica Acta
化学-分析化学
CiteScore
10.40
自引率
6.50%
发文量
1081
审稿时长
38 days
期刊介绍:
Analytica Chimica Acta has an open access mirror journal Analytica Chimica Acta: X, sharing the same aims and scope, editorial team, submission system and rigorous peer review.
Analytica Chimica Acta provides a forum for the rapid publication of original research, and critical, comprehensive reviews dealing with all aspects of fundamental and applied modern analytical chemistry. The journal welcomes the submission of research papers which report studies concerning the development of new and significant analytical methodologies. In determining the suitability of submitted articles for publication, particular scrutiny will be placed on the degree of novelty and impact of the research and the extent to which it adds to the existing body of knowledge in analytical chemistry.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


