Synthesis, in vitro and in vivo biological evaluation of novel dual compounds targeting both acetylcholinesterase and serotonergic 5-HT4 receptors with potential interest in the treatment of Alzheimer's disease
IF 6
2区 医学
Q1 CHEMISTRY, MEDICINAL
引用次数: 0
Abstract
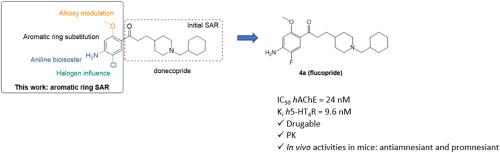
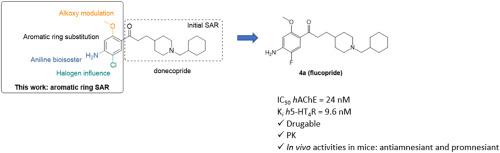
In this work, we exemplified the “copride” family of drug candidates able to both inhibit acetylcholinesterase and to activate 5-HT4 receptors, with anti-amnesiant and promnesiant activities in mice. Twenty-one analogs of donecopride, the first-in class representative of the series, were synthesized exploring the influence on the biological activities of the substituents (methoxy, amine and chlorine) carried by its phenyl ring. This work was the support of an intensive structure-activity relationship study and allowed to obtain some interesting derivatives of donecopride. In this respect, the replacement of the methoxy group of the latter with a deuterated one led to deudonecopride. On the other hand, the replacement of the chlorine atom of donecopride by various halogen atoms was of particular interest, among which fluorine led to a potent analog, we called flucopride. The latter exhibited promising in vitro activities associated to excellent drugability parameters. Flucopride was consequently involved in in vivo studies such as a scopolamine-induced deficit model of working memory and in a novel object recognition test. Through these evaluations, flucopride demonstrated both its antiamnesiant and promnesiant capacities, which could make it a potential preclinical drug candidate for the treatment of Alzheimer's disease.
同时靶向乙酰胆碱酯酶和羟色胺能 5-HT4 受体的新型双效化合物的合成、体外和体内生物学评估,有望用于治疗阿尔茨海默病。
在这项工作中,我们举例说明了 "copride "系列候选药物,它们既能抑制乙酰胆碱酯酶,又能激活 5-HT4 受体,对小鼠具有抗失忆和促失忆活性。我们合成了 21 种多奈必利的类似物,它们是该系列药物中的首个同类代表,研究了其苯环上的取代基(甲氧基、胺和氯)对生物活性的影响。这项工作为深入的结构-活性关系研究提供了支持,并获得了一些有趣的多奈克必利衍生物。在这方面,用氚化基团取代后者的甲氧基,可以得到多酮必利。另一方面,用各种卤素原子取代多奈氯必利的氯原子也特别令人感兴趣,其中氟原子导致了一种强效类似物,我们称之为氟氯必利。后者表现出良好的体外活性和出色的可药性参数。因此,氟氯必利被用于体内研究,如东莨菪碱诱导的工作记忆缺陷模型和新型物体识别测试。通过这些评估,氟氯必利证明了其抗失忆和促失忆的能力,这可能使其成为治疗阿尔茨海默病的临床前候选药物。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
11.70
自引率
9.00%
发文量
863
审稿时长
29 days
期刊介绍:
The European Journal of Medicinal Chemistry is a global journal that publishes studies on all aspects of medicinal chemistry. It provides a medium for publication of original papers and also welcomes critical review papers.
A typical paper would report on the organic synthesis, characterization and pharmacological evaluation of compounds. Other topics of interest are drug design, QSAR, molecular modeling, drug-receptor interactions, molecular aspects of drug metabolism, prodrug synthesis and drug targeting. The journal expects manuscripts to present the rational for a study, provide insight into the design of compounds or understanding of mechanism, or clarify the targets.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


