Naphthalen-1-ylethanamine–containing small molecule inhibitors of the papain-like protease of SARS-CoV-2
IF 6
2区 医学
Q1 CHEMISTRY, MEDICINAL
引用次数: 0
Abstract
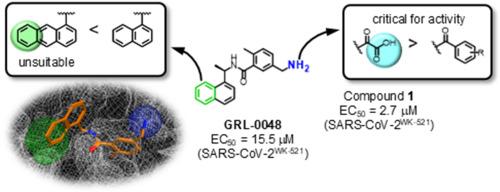
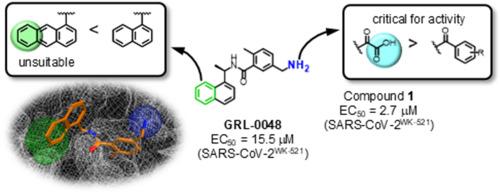
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), which causes coronavirus disease 2019 (COVID-19), has not yet been eradicated. SARS-CoV-2 has two types of proteases, a main protease (Mpro) and a papain-like protease (PLpro), which together process two translated non-structural polyproteins, pp1a and pp1ab, to produce functional viral proteins. In this study, effective inhibitors against PLpro of SARS-CoV-2 were designed and synthesized using GRL-0048 as a lead. A docking simulation of GRL-0048 and SARS-CoV-2 PLpro showed that GRL-0048 noncovalently interacts with PLpro, and there is a newly identified binding pocket in PLpro. Structure-activity relationship studies were next performed on GRL-0048, resulting in the development of several inhibitors, specifically compounds 1, 2b, and 3h, that have more potent inhibitory activity than GRL-0048.
含萘-1-乙胺的 SARS-CoV-2 木瓜蛋白酶类小分子抑制剂
导致 2019 年冠状病毒病(COVID-19)的严重急性呼吸系统综合征冠状病毒 2(SARS-CoV-2)尚未被根除。SARS-CoV-2 有两种蛋白酶,一种是主蛋白酶(Mpro),另一种是木瓜蛋白酶样蛋白酶(PLpro),它们共同处理两种已翻译的非结构性多聚蛋白 pp1a 和 pp1ab,生成功能性病毒蛋白。本研究以 GRL-0048 为先导,设计并合成了针对 SARS-CoV-2 PLpro 的有效抑制剂。GRL-0048与SARS-CoV-2 PLpro的对接模拟显示,GRL-0048与PLpro存在非共价相互作用,并且在PLpro中发现了一个新的结合口袋。接下来对 GRL-0048 进行了结构-活性关系研究,最终开发出了几种抑制剂,特别是化合物 1、2b 和 3h,它们比 GRL-0048 具有更强的抑制活性。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
11.70
自引率
9.00%
发文量
863
审稿时长
29 days
期刊介绍:
The European Journal of Medicinal Chemistry is a global journal that publishes studies on all aspects of medicinal chemistry. It provides a medium for publication of original papers and also welcomes critical review papers.
A typical paper would report on the organic synthesis, characterization and pharmacological evaluation of compounds. Other topics of interest are drug design, QSAR, molecular modeling, drug-receptor interactions, molecular aspects of drug metabolism, prodrug synthesis and drug targeting. The journal expects manuscripts to present the rational for a study, provide insight into the design of compounds or understanding of mechanism, or clarify the targets.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


