Dizocilpine derivatives as neuroprotective NMDA receptor antagonists without psychomimetic side effects
IF 6
2区 医学
Q1 CHEMISTRY, MEDICINAL
引用次数: 0
Abstract
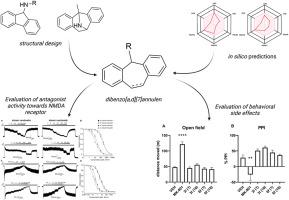
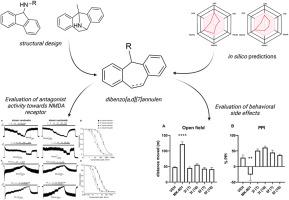
We aimed to prepare novel dibenzo [a,d][7]annulen derivatives that act on N-methyl-d-aspartate (NMDA) receptors with potential neuroprotective effects. Our approach involved modifying the tropane moiety of MK-801, a potent open-channel blocker known for its psychomimetic side effects, by introducing a seven-membered ring with substituted base moieties specifically to alleviate these undesirable effects. Our in silico analyses showed that these derivatives should have high gastrointestinal absorption and cross the blood-brain barrier (BBB). Our pharmacokinetic studies in rats supported this conclusion and confirmed the ability of leading compounds 3l and 6f to penetrate the BBB. Electrophysiological experiments showed that all compounds exhibited different inhibitory activity towards the two major NMDA receptor subtypes, GluN1/GluN2A and GluN1/GluN2B. Of the selected compounds intentionally differing in the inhibitory efficacy, 6f showed high relative inhibition (∼90 % for GluN1/GluN2A), while 3l showed moderate inhibition (∼50 %). An in vivo toxicity study determined that compounds 3l and 6f were safe at 10 mg/kg doses with no adverse effects. Behavioral studies demonstrated that these compounds did not induce hyperlocomotion or impair prepulse inhibition of startle response in rats. Neuroprotective assays using a model of NMDA-induced hippocampal neurodegeneration showed that compound 3l at a concentration of 30 μM significantly reduced hippocampal damage in rats. These results suggest that these novel dibenzo [a,d][7]annulen derivatives are promising candidates for developing NMDA receptor-targeted therapies with minimal psychotomimetic side effects.
作为神经保护性 NMDA 受体拮抗剂的地佐西平衍生物不会产生拟精神副作用
我们的目标是制备新型二苯并[a,d][7]茚衍生物,这种衍生物可作用于 N-甲基-D-天冬氨酸(NMDA)受体,具有潜在的神经保护作用。MK-801是一种强效开放通道阻断剂,以其拟精神副作用而闻名,我们的研究方法是通过引入一个具有取代基分子的七元环,对MK-801的托烷分子进行修饰,以减轻这些不良反应。我们的硅学分析表明,这些衍生物应具有较高的胃肠道吸收率,并能穿过血脑屏障(BBB)。我们在大鼠体内进行的药代动力学研究支持了这一结论,并证实了前导化合物 3l 和 6f 穿透 BBB 的能力。电生理实验表明,所有化合物都对两种主要的 NMDA 受体亚型(GluN1/GluN2A 和 GluN1/GluN2B)具有不同的抑制活性。在所选的抑制效力有意不同的化合物中,6f 表现出较高的相对抑制作用(对 GluN1/GluN2A 的抑制率为 90%∼),而 3l 则表现出中等程度的抑制作用(50%∼)。一项体内毒性研究确定,化合物 3l 和 6f 在 10 毫克/千克剂量下是安全的,没有不良反应。行为研究表明,这些化合物不会诱发大鼠过度运动,也不会损害对惊吓反应的前脉冲抑制。使用 NMDA 诱导的海马神经变性模型进行的神经保护试验表明,浓度为 30 μM 的化合物 3l 能显著减轻大鼠海马的损伤。这些结果表明,这些新型二苯并[a,d][7]茚衍生物是开发 NMDA 受体靶向疗法的有希望的候选化合物,其拟精神药物的副作用极小。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
11.70
自引率
9.00%
发文量
863
审稿时长
29 days
期刊介绍:
The European Journal of Medicinal Chemistry is a global journal that publishes studies on all aspects of medicinal chemistry. It provides a medium for publication of original papers and also welcomes critical review papers.
A typical paper would report on the organic synthesis, characterization and pharmacological evaluation of compounds. Other topics of interest are drug design, QSAR, molecular modeling, drug-receptor interactions, molecular aspects of drug metabolism, prodrug synthesis and drug targeting. The journal expects manuscripts to present the rational for a study, provide insight into the design of compounds or understanding of mechanism, or clarify the targets.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


