Programmable bacteria synergize with PD-1 blockade to overcome cancer cell–intrinsic immune resistance mechanisms
IF 17.6
1区 医学
Q1 IMMUNOLOGY
引用次数: 0
Abstract
Interferon-γ (IFN-γ) is a potent cytokine critical for response to immunotherapy, yet conventional methods to systemically deliver this cytokine have been hindered by severe dose-limiting toxicities. Here, we engineered a strain of probiotic bacteria that home to tumors and locally release IFN-γ. A single intratumoral injection of these IFN-γ–producing bacteria was sufficient to drive systemic tumor antigen–specific antitumor immunity, without observable toxicity. Although cancer cells use various resistance mechanisms to evade immune responses, bacteria-derived IFN-γ overcame primary resistance to programmed cell death 1 (PD-1) blockade via activation of cytotoxic Foxp3−CD4+ and CD8+ T cells. Moreover, by activating natural killer (NK) cells, bacteria-derived IFN-γ also overcame acquired resistance mechanisms to PD-1 blockade, specifically loss-of-function mutations in IFN-γ signaling and antigen presentation pathways. Collectively, these results demonstrate the promise of combining IFN-γ–producing bacteria with PD-1 blockade as a therapeutic strategy for overcoming immunotherapy-resistant, locally advanced, and metastatic disease.
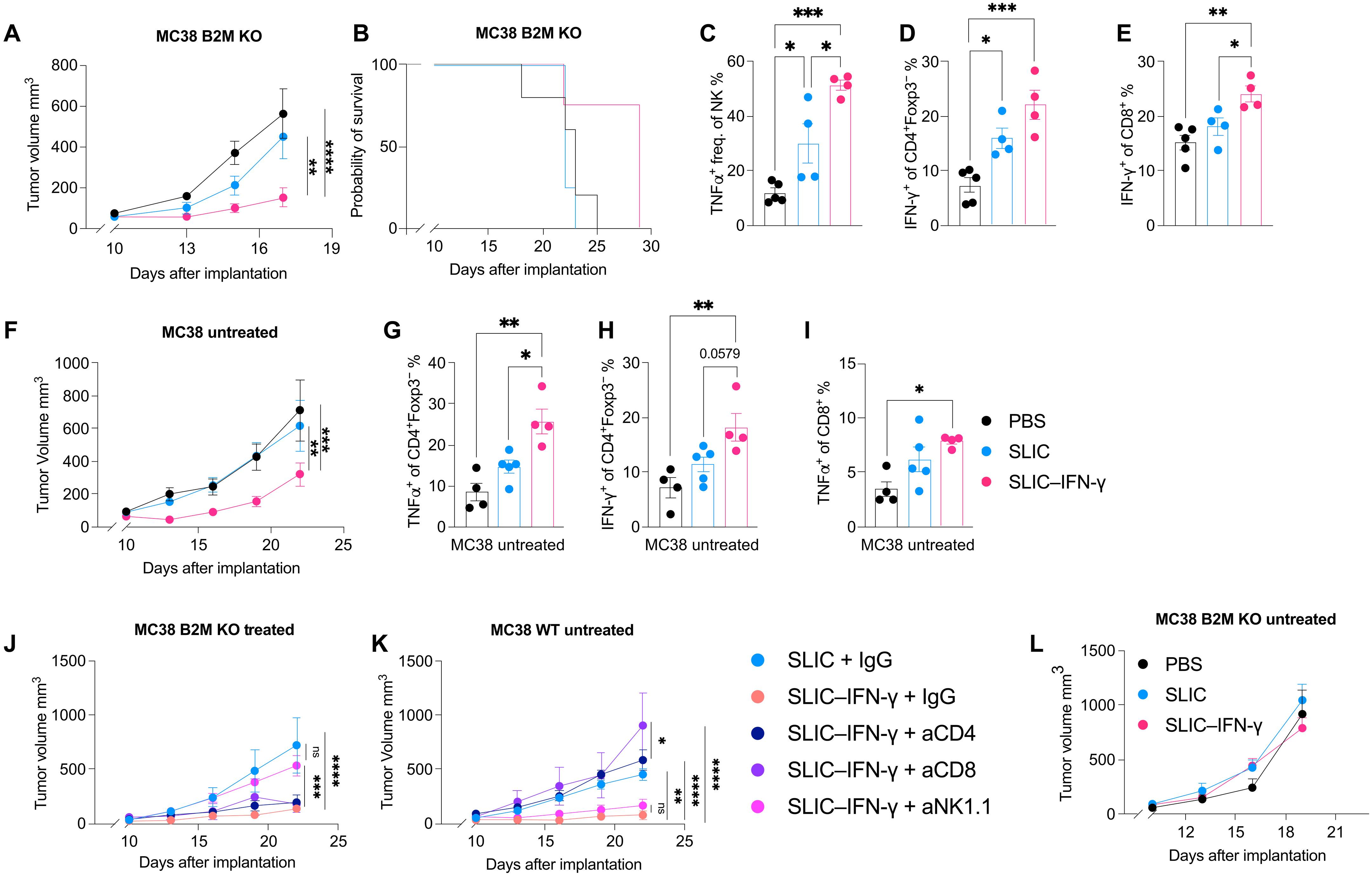
可编程细菌与 PD-1 阻断剂协同作用,克服癌细胞内在的免疫抵抗机制
干扰素-γ(IFN-γ)是一种强效细胞因子,对免疫疗法的反应至关重要,但系统性释放这种细胞因子的传统方法一直受到严重剂量限制毒性的阻碍。在这里,我们设计了一株益生菌,它能在肿瘤局部释放 IFN-γ。只需在肿瘤内注射一次这些产生IFN-γ的细菌,就足以驱动全身性的肿瘤抗原特异性抗肿瘤免疫,且无明显毒性。虽然癌细胞利用各种抵抗机制来逃避免疫反应,但细菌产生的 IFN-γ 通过激活细胞毒性 Foxp3 - CD4 + 和 CD8 + T 细胞,克服了对程序性细胞死亡 1(PD-1)阻断的主要抵抗。此外,通过激活自然杀伤(NK)细胞,细菌衍生的 IFN-γ 还克服了 PD-1 阻断的获得性抗性机制,特别是 IFN-γ 信号传导和抗原递呈通路中的功能缺失突变。总之,这些结果表明,将产生IFN-γ的细菌与PD-1阻断结合起来,作为一种治疗策略,有望克服免疫治疗耐药、局部晚期和转移性疾病。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Science Immunology
Immunology and Microbiology-Immunology
CiteScore
32.90
自引率
2.00%
发文量
183
期刊介绍:
Science Immunology is a peer-reviewed journal that publishes original research articles in the field of immunology. The journal encourages the submission of research findings from all areas of immunology, including studies on innate and adaptive immunity, immune cell development and differentiation, immunogenomics, systems immunology, structural immunology, antigen presentation, immunometabolism, and mucosal immunology. Additionally, the journal covers research on immune contributions to health and disease, such as host defense, inflammation, cancer immunology, autoimmunity, allergy, transplantation, and immunodeficiency. Science Immunology maintains the same high-quality standard as other journals in the Science family and aims to facilitate understanding of the immune system by showcasing innovative advances in immunology research from all organisms and model systems, including humans.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


