Leveraging Atomic-Scale Synergy for Selective CO2 Electrocatalysis to CO over CuNi Dual-Atom Catalysts
IF 11.3
1区 化学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
Revealing the synergistic catalytic mechanism involving multiple active centers is crucial for understanding multiphase catalysis. However, the complex structures of catalysts and interfacial environments pose a challenge in thoroughly exploring the experimental evidence. This study reports the utilization of a CuNi dual-atom catalyst (Cu/Ni–NC) for the electrochemical reduction of CO2. It demonstrates a high Faradaic efficiency of CO exceeding 99%, remarkable reaction activity with a partial current density surpassing –300 mA cm–2, and prolonged stability for more than 5 days at a current density of –200 mA·cm–2. Operando characterization techniques and density functional theory calculations reveal that Ni atoms function as active sites for the activation and hydrogenation of CO2, while Cu atoms serve as active sites for the dissociation of H2O, supplying protons for the subsequent hydrogenation process. Moreover, the electronic interactions between Ni and Cu atoms facilitate the formation of *COOH and the dissociation of H2O, illustrating a synergistic reduction of CO2 at the dual-atom sites.
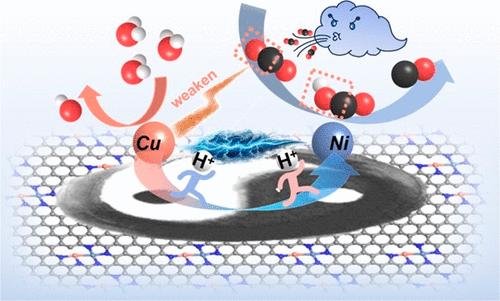
利用原子尺度的协同作用,在铜镍双原子催化剂上进行选择性 CO2 电催化制 CO
揭示涉及多个活性中心的协同催化机制对于理解多相催化至关重要。然而,催化剂和界面环境的复杂结构给深入探索实验证据带来了挑战。本研究报告了利用铜镍双原子催化剂(Cu/Ni-NC)电化学还原 CO2 的情况。该催化剂对一氧化碳的法拉第效率高达 99% 以上,反应活性显著,部分电流密度超过 -300 mA cm-2,并且在电流密度为 -200 mA-cm-2 的条件下可保持 5 天以上的稳定性。运算表征技术和密度泛函理论计算表明,镍原子是活化和氢化二氧化碳的活性位点,而铜原子则是解离 H2O 的活性位点,为随后的氢化过程提供质子。此外,镍原子和铜原子之间的电子相互作用促进了*COOH 的形成和 H2O 的解离,说明了在双原子位点上 CO2 的协同还原作用。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

ACS Catalysis
CHEMISTRY, PHYSICAL-
CiteScore
20.80
自引率
6.20%
发文量
1253
审稿时长
1.5 months
期刊介绍:
ACS Catalysis is an esteemed journal that publishes original research in the fields of heterogeneous catalysis, molecular catalysis, and biocatalysis. It offers broad coverage across diverse areas such as life sciences, organometallics and synthesis, photochemistry and electrochemistry, drug discovery and synthesis, materials science, environmental protection, polymer discovery and synthesis, and energy and fuels.
The scope of the journal is to showcase innovative work in various aspects of catalysis. This includes new reactions and novel synthetic approaches utilizing known catalysts, the discovery or modification of new catalysts, elucidation of catalytic mechanisms through cutting-edge investigations, practical enhancements of existing processes, as well as conceptual advances in the field. Contributions to ACS Catalysis can encompass both experimental and theoretical research focused on catalytic molecules, macromolecules, and materials that exhibit catalytic turnover.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


