Catalytic asymmetric Michael and Nef-type sequential reaction of nitroolefin with azlactone to construct oxazole-fused succinimide†
IF 4.3
2区 化学
Q2 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
A series of oxazole-fused succinimides bearing vicinal quaternary carbon centers were synthesized. This process takes place between nitroolefins and azlactones in the presence of a bifunctional chiral guanidine-sulfonamide organocatalyst, followed by a Nef-type transformation under the treatment of DMAP/Ac2O. Several control experiments were conducted to propose the mechanism.
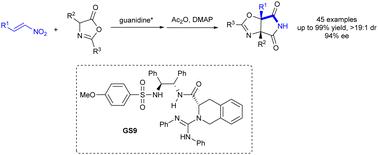
硝基烯烃与氮杂环丁内酯的催化不对称迈克尔和 Nef 型顺序反应生成草唑融合琥珀酰亚胺
我们合成了一系列带有邻接季碳中心的噁唑融合琥珀酰亚胺。该过程在双功能手性胍-磺酰胺有机催化剂存在下发生在硝基烯烃和氮内酯之间,然后在 DMAP/Ac2O 处理下发生 Nef 型转化。为了提出这一机理,进行了一些对照实验。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Chemical Communications
化学-化学综合
CiteScore
8.60
自引率
4.10%
发文量
2705
审稿时长
1.4 months
期刊介绍:
ChemComm (Chemical Communications) is renowned as the fastest publisher of articles providing information on new avenues of research, drawn from all the world''s major areas of chemical research.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


