Immunogenicity risk assessment and mitigation for engineered antibody and protein therapeutics
IF 101.8
1区 医学
Q1 BIOTECHNOLOGY & APPLIED MICROBIOLOGY
引用次数: 0
Abstract
Remarkable progress has been made in recent decades in engineering antibodies and other protein therapeutics, including enhancements to existing functions as well as the advent of novel molecules that confer biological activities previously unknown in nature. These protein therapeutics have brought major benefits to patients across multiple areas of medicine. One major ongoing challenge is that protein therapeutics can elicit unwanted immune responses (immunogenicity) in treated patients, including the generation of anti-drug antibodies. In rare and unpredictable cases, anti-drug antibodies can seriously compromise therapeutic safety and/or efficacy. Systematic deconvolution of this immunogenicity problem is confounded by the complexity of its many contributing factors and the inherent limitations of available experimental and computational methods. Nevertheless, continued progress with the assessment and mitigation of immunogenicity risk at the preclinical stage has the potential to reduce the incidence and severity of clinical immunogenicity events. This Review focuses on identifying key unsolved anti-drug antibody-related challenges and offers some pragmatic approaches towards addressing them. Examples are drawn mainly from antibodies, given that the majority of available clinical data are from this class of protein therapeutics. Plausible and seemingly tractable solutions are in sight for some immunogenicity problems, whereas other challenges will likely require completely new approaches. Engineered protein therapeutics, including antibodies, are valuable drugs offering major health benefits, but they can elicit unwanted immune responses. This Review identifies key challenges in assessing and mitigating the risk of immunogenicity, particularly the generation of anti-drug antibodies, and suggests pragmatic steps to address them.
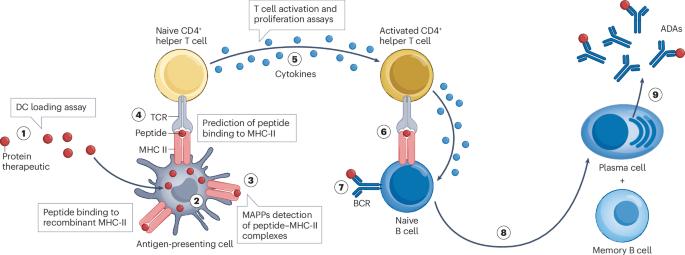
工程抗体和蛋白质疗法的免疫原性风险评估与缓解
近几十年来,在抗体和其他蛋白质疗法的工程设计方面取得了显著进展,包括增强现有功能,以及出现赋予生物活性的新型分子,而这些生物活性以前在自然界中是未知的。这些蛋白质疗法为多个医学领域的患者带来了重大益处。目前面临的一个主要挑战是,蛋白质疗法可能会在接受治疗的患者中引起不必要的免疫反应(免疫原性),包括产生抗药性抗体。在罕见和不可预测的情况下,抗药性抗体会严重影响治疗的安全性和/或疗效。由于导致免疫原性的因素众多且复杂,而现有的实验和计算方法又存在固有的局限性,因此系统性地解决这一免疫原性问题受到了限制。不过,在临床前阶段评估和降低免疫原性风险方面的持续进展有可能降低临床免疫原性事件的发生率和严重程度。本综述侧重于确定尚未解决的抗药抗体相关关键挑战,并提供一些务实的方法来应对这些挑战。鉴于大多数可用的临床数据都来自抗体这一类蛋白质疗法,因此本综述主要从抗体方面举例说明。对于一些免疫原性问题,我们看到了看似可行的解决方案,而其他挑战则可能需要全新的方法。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature Reviews. Drug Discovery
医学-生物工程与应用微生物
CiteScore
137.40
自引率
0.30%
发文量
227
期刊介绍:
Nature Reviews Drug Discovery is a monthly journal aimed at everyone working in the drug discovery and development arena.
Each issue includes:
Highest-quality reviews and perspectives covering a broad scope.
News stories investigating the hottest topics in drug discovery.
Timely summaries of key primary research papers.
Concise updates on the latest advances in areas such as new drug approvals, patent law, and emerging industry trends and strategies.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


