Serinc2 Drives the Progression of Cervical Cancer Through Regulating Myc Pathway
Abstract
Background
As one of the most common malignancies, cervical cancer (CC) seriously affects women's health. This study aimed to investigate the biological function of Serinc2 in CC.
Methods
Serinc2 expression was surveyed utilizing immunohistochemistry, western blot, and qRT-PCR. CC cell viability, invasion, proliferation, migration, and apoptosis, were detected via CCK-8, Transwell assay, colony formation, wound healing assay, and flow cytometry. Glucose consumption, lactate production, and ATP levels were determined by the corresponding kit. The protein expression of c-Myc, PDK1, HK2, PFKP, LDHA, Snail, Vimentin, N-cadherin, and E-cadherin was detected via western blot. The interaction between the promoter of PFKP and Myc was confirmed through luciferase reporter assay and Chip assay. In vivo, to evaluate the function of Serinc2 on tumor growth, a xenograft mouse model was used.
Results
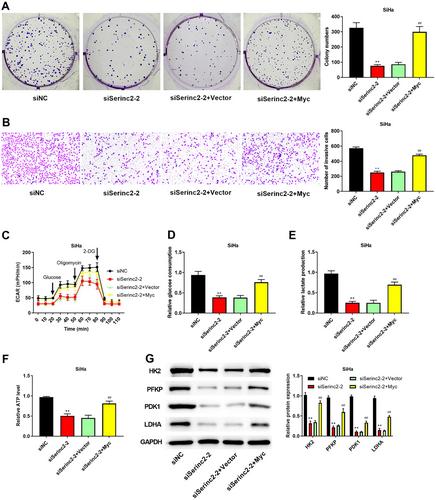
In CC tissues and cells, Serinc2 was upregulated. In CC cells, knockdown of Serinc2 suppressed cell invasion, proliferation, migration, decreased the expression of Snail, Vimentin, N-cadherin, HK2, PFKP, LDHA, and PDK1, increased E-cadherin expression, reduced glucose consumption and the production of lactate and ATP, and induced cell apoptosis; Serinc2 overexpression led to the opposite results. Mechanically, Serinc2 promoted Myc expression, and Myc induced PFKP expression. Furthermore, overexpressed Myc abolished the inhibitive influences of Serinc2 knockdown on the malignant behaviors of CC cells. Additionally, knockdown of Serinc2 inhibited tumor growth and reduced the protein expression of c-Myc, PFKP, LDHA, and PDK1 in vivo.
Conclusions
Knockdown of Serinc2 inhibited the malignant progression of CC, which was achieved via Myc pathway. Our study provides novel insight into CC pathogenesis.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


