Efficient low-temperature CO removal from high-humidity sintering flue gas by combination Cu and OMS-2
IF 4.8
3区 材料科学
Q1 CHEMISTRY, APPLIED
引用次数: 0
Abstract
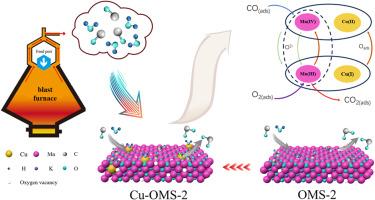
The non-precious metal manganese-based catalysts currently show insufficient CO catalytic oxidation performance in actual sintering flue gas conditions, with increased water vapor levels causing catalyst deactivation. This study utilized a high-performance manganese dioxide octahedral molecular sieve (OMS-2). The effect of metal doping on the catalytic CO oxidation performance was investigated in preparing OMS-2 using the co-precipitation method. The experiments showed that Cu doping increased CO conversion efficiency more than other metals (Co, Ag, Zn, and Fe), with optimal performance achieved at a 1.91 wt% doping level. Besides, Cu doping significantly enhanced water resistance of the catalyst, enabling effective CO removal in high-humidity conditions. The study observed that Cu ions infiltrated the catalyst framework by substituting some of the Mn ions, creating additional active sites in the form of oxygen vacancies and improving surface oxygen mobility, thereby enhancing the performance of CO catalytic oxidation. Furthermore, Cu doping demonstrated selective absorption of water vapor, with CuxO in the catalyst, effectively adsorbing water vapor and protecting the initial active sites, thereby mitigating water vapor-induced poisoning. Even in 15 vol% H2O at 150 °C, 1.91%Cu-OMS-2 maintained total catalytic activity. Therefore, the co-precipitation method-prepared 1.91%Cu-OMS-2 catalyst holds excellent potential for CO removal in sintering flue gas and shows promise for practical applications.
利用 Cu 和 OMS-2 组合高效去除高湿度烧结烟气中的低温 CO
目前,非贵金属锰基催化剂在实际烧结烟气条件下的一氧化碳催化氧化性能不足,水蒸气含量增加会导致催化剂失活。本研究采用了高性能八面体分子筛二氧化锰(OMS-2)。在使用共沉淀法制备 OMS-2 的过程中,研究了金属掺杂对催化 CO 氧化性能的影响。实验结果表明,与其他金属(Co、Ag、Zn 和 Fe)相比,掺杂 Cu 更能提高 CO 的转化效率,在 1.91 wt% 的掺杂水平下达到最佳性能。此外,掺杂铜还能显著提高催化剂的耐水性,从而在高湿度条件下有效去除一氧化碳。研究观察到,Cu 离子通过取代部分 Mn 离子渗入催化剂框架,以氧空位的形式产生了额外的活性位点,并改善了表面氧的流动性,从而提高了 CO 催化氧化的性能。此外,催化剂中的 CuxO 可选择性地吸收水蒸气,有效地吸附了水蒸气并保护了初始活性位点,从而减轻了水蒸气引起的中毒。即使在 150 °C、15 vol% H2O 的条件下,1.91%Cu-OMS-2 也能保持完全的催化活性。因此,共沉淀法制备的 1.91%Cu-OMS-2 催化剂在烧结烟气中脱除 CO 方面具有很好的潜力,有望得到实际应用。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Microporous and Mesoporous Materials
化学-材料科学:综合
CiteScore
10.70
自引率
5.80%
发文量
649
审稿时长
26 days
期刊介绍:
Microporous and Mesoporous Materials covers novel and significant aspects of porous solids classified as either microporous (pore size up to 2 nm) or mesoporous (pore size 2 to 50 nm). The porosity should have a specific impact on the material properties or application. Typical examples are zeolites and zeolite-like materials, pillared materials, clathrasils and clathrates, carbon molecular sieves, ordered mesoporous materials, organic/inorganic porous hybrid materials, or porous metal oxides. Both natural and synthetic porous materials are within the scope of the journal.
Topics which are particularly of interest include:
All aspects of natural microporous and mesoporous solids
The synthesis of crystalline or amorphous porous materials
The physico-chemical characterization of microporous and mesoporous solids, especially spectroscopic and microscopic
The modification of microporous and mesoporous solids, for example by ion exchange or solid-state reactions
All topics related to diffusion of mobile species in the pores of microporous and mesoporous materials
Adsorption (and other separation techniques) using microporous or mesoporous adsorbents
Catalysis by microporous and mesoporous materials
Host/guest interactions
Theoretical chemistry and modelling of host/guest interactions
All topics related to the application of microporous and mesoporous materials in industrial catalysis, separation technology, environmental protection, electrochemistry, membranes, sensors, optical devices, etc.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


