Exosomes from hypoxic urine-derived stem cells facilitate healing of diabetic wound by targeting SERPINE1 through miR-486-5p
IF 12.9
1区 医学
Q1 ENGINEERING, BIOMEDICAL
引用次数: 0
Abstract
Vascular pathologies and injuries are important factors for the delayed wound healing in diabetes. Previous studies have demonstrated that hypoxic environments could induce formation of new blood vessels by regulating intercellular communication and cellular behaviors. In this study, we have enhanced the angiogenic potential of exosomes by subjecting urine-derived stem cells (USCs) to hypoxic preconditioning. To prolong the retention of exosomes at the wound site, we have also engineered a novel dECM hydrogel termed SISMA, which was modified from porcine small intestinal submucosa (SIS). For its rapid and controllable gelation kinetics, excellent biocompatibility, and exosome release capability, the SISMA hydrogel has proven to be a reliable delivery vehicle for exosomes. The hypoxia-induced exosomes-loaded hydrogel has promoted endothelial cell proliferation, migration, and tube formation. More importantly, as evidenced by significant in vivo vascular regeneration in the early stages post-injury, it has facilitated tissue repair. This may because miR-486–5p in H-exo inhibit SERPINE1 activity in endothelial cell. Additionally, miRNA sequencing analysis suggested that the underlying mechanism for enhanced angiogenesis may be associated with the activation of classical HIF-1α signaling pathway. In summary, our study has presented a novel non-invasive, cell-free therapeutic approach for accelerating diabetes wound healing and development of a practical and efficient exosomes delivery platform.
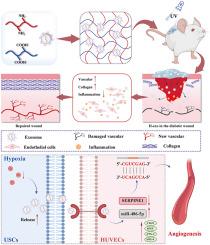
缺氧尿源干细胞的外泌体通过 miR-486-5p 靶向 SERPINE1 促进糖尿病伤口愈合
血管病变和损伤是糖尿病患者伤口愈合延迟的重要因素。以往的研究表明,缺氧环境可通过调节细胞间的交流和细胞行为诱导新血管的形成。在这项研究中,我们通过对尿源性干细胞(USCs)进行缺氧预处理,增强了外泌体的血管生成潜能。为了延长外泌体在伤口部位的滞留时间,我们还设计了一种新型 dECM 水凝胶,称为 SISMA,它是由猪小肠粘膜下层(SIS)改性而成的。SISMA 水凝胶具有快速可控的凝胶化动力学、良好的生物相容性和外泌体释放能力,已被证明是一种可靠的外泌体输送载体。缺氧诱导的外泌体负载水凝胶促进了内皮细胞的增殖、迁移和管道形成。更重要的是,在损伤后的早期阶段,体内血管再生显著,证明它促进了组织修复。这可能是因为 H-exo 中的 miR-486-5p 抑制了内皮细胞中 SERPINE1 的活性。此外,miRNA 测序分析表明,血管生成增强的潜在机制可能与经典 HIF-1α 信号通路的激活有关。总之,我们的研究提出了一种新的非侵入性、无细胞治疗方法,可用于加速糖尿病伤口愈合,并开发了一种实用、高效的外泌体递送平台。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Biomaterials
工程技术-材料科学:生物材料
CiteScore
26.00
自引率
2.90%
发文量
565
审稿时长
46 days
期刊介绍:
Biomaterials is an international journal covering the science and clinical application of biomaterials. A biomaterial is now defined as a substance that has been engineered to take a form which, alone or as part of a complex system, is used to direct, by control of interactions with components of living systems, the course of any therapeutic or diagnostic procedure. It is the aim of the journal to provide a peer-reviewed forum for the publication of original papers and authoritative review and opinion papers dealing with the most important issues facing the use of biomaterials in clinical practice. The scope of the journal covers the wide range of physical, biological and chemical sciences that underpin the design of biomaterials and the clinical disciplines in which they are used. These sciences include polymer synthesis and characterization, drug and gene vector design, the biology of the host response, immunology and toxicology and self assembly at the nanoscale. Clinical applications include the therapies of medical technology and regenerative medicine in all clinical disciplines, and diagnostic systems that reply on innovative contrast and sensing agents. The journal is relevant to areas such as cancer diagnosis and therapy, implantable devices, drug delivery systems, gene vectors, bionanotechnology and tissue engineering.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


