Shikonin induces ferroptosis in osteosarcomas through the mitochondrial ROS-regulated HIF-1α/HO-1 axis
IF 6.7
1区 医学
Q1 CHEMISTRY, MEDICINAL
引用次数: 0
Abstract
Background
The most common malignant bone tumour is osteosarcoma, which has an unsatisfactory prognosis and unsatisfactory treatment. Ferroptosis shows promise as an effective OS therapy. A substance extracted from the Lithospermum erythrorhizon, Shikonin (SHK), inhibits a number of tumours, including ovarian, gastric, and lung cancers. However, whether SHK induces OS ferroptosis and its mechanisms are not clear.
Purpose
Our study is aimed at investigating whether SHK causes ferroptosis and elucidating its molecular mechanism.
Methods
Cell counting Kit-8, cell cycle and cell apoptosis assay were utilised to assess therapeutic effect of SHK against OS. Normal cells, including H9C2, AML-12 and HK-2, haematoxylin and eosin staining and mice serum biomarker tests were used to assess SHK biosafety. Malondialdehyde (MDA) levels, the glutathione (GSH)/oxidized glutathione (GSSG) ratio, reactive oxygen species (ROS), lipid peroxide (LPO), and intracellular Fe2+ detection, quantitative reverse transcription PCR (qRT-PCR), Western blotting (WB) and rescue experiments were employed to confirm whether SHK induced ferroptosis in OS cells. Molecular docking, cellular thermal shift assay (CETSA), and drug affinity responsive target stability (DARTS) assay were used to evaluate the direct binding between SHK and hypoxia-inducible factor-1α (HIF-1α). Protein stability and degradation analysis, small RNA interference, flow cytometry, qRT-PCR, and WB were used to investigate the molecular mechanism of ferroptosis. In vivo xenografts of nude mouse was used to study the anti-OS effect of SHK.
Results
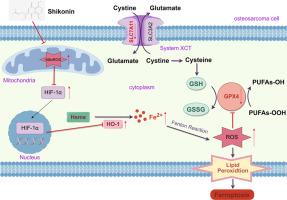
SHK significantly reduced OS cell viability and induced apoptosis and G2/M arrest. SHK increased intracellular levels of MDA, ROS, LPO, and Fe2+ while simultaneously reducing the GSH/GSSG ratio and GPX4 and SLC7A11 expression. CETSA and DARTS results demonstrated that SHK did not bind targetly to HIF-1α. Instead, mitochondrial ROS (MitoROS) promoted HIF-1α expression, resulting in HO-1 overexpression, excess Fe2+ production, ROS accumulation and GPX depletion, and ferroptosis. Furthermore, inhibition of MitoROS using Mito-TEMPO downregulated HIF-1α/HO-1 axis and mitigated the SHK-induced ferroptosis. In vivo, SHK effectively suppressed OS growth with favourable biosafety, confirming the molecular mechanism underlying SHK-induced ferroptosis in OS.
Conclusion
We observe that HIF-1α/HO-1 axis is the crucial factor in SHK-induced OS ferroptosis. Importantly, we demonstrate that HIF-1α is indirectly regulated by MitoROS rather than SHK bound directly to HIF-1α. Our research suggest that SHK is a potential drug candidate for OS treatment and may help in identifying novel therapeutic targets for OS.
志贺宁通过线粒体 ROS 调控的 HIF-1α/HO-1 轴诱导骨肉瘤中的铁变态反应
背景最常见的恶性骨肿瘤是骨肉瘤,其预后不佳,治疗效果也不理想。作为一种有效的骨肉瘤治疗方法,铁氧体拮抗剂具有广阔的前景。从红豆杉(Lithospermum erythrorhizon)中提取的一种物质--Shikonin(SHK)能抑制多种肿瘤,包括卵巢癌、胃癌和肺癌。目的 我们的研究旨在探讨 SHK 是否会导致铁细胞凋亡,并阐明其分子机制。方法 利用细胞计数 Kit-8、细胞周期和细胞凋亡检测来评估 SHK 对 OS 的治疗效果。利用正常细胞(包括 H9C2、AML-12 和 HK-2)、血栓素和伊红染色以及小鼠血清生物标记物检测来评估 SHK 的生物安全性。采用丙二醛(MDA)水平、谷胱甘肽(GSH)/氧化谷胱甘肽(GSSG)比率、活性氧(ROS)、过氧化脂质(LPO)和细胞内Fe2+检测、定量反转录PCR(qRT-PCR)、Western印迹(WB)和拯救实验来证实SHK是否诱导OS细胞的铁变态反应。分子对接、细胞热转移试验(CETSA)和药物亲和力反应靶点稳定性试验(DARTS)评估了SHK与缺氧诱导因子-1α(HIF-1α)的直接结合。蛋白质稳定性和降解分析、小 RNA 干扰、流式细胞术、qRT-PCR 和 WB 被用来研究铁变态反应的分子机制。结果SHK能显著降低OS细胞活力,诱导细胞凋亡和G2/M停滞。SHK增加了细胞内MDA、ROS、LPO和Fe2+的水平,同时降低了GSH/GSSG比率以及GPX4和SLC7A11的表达。CETSA 和 DARTS 结果表明,SHK 并不与 HIF-1α 靶向结合。相反,线粒体 ROS(MitoROS)会促进 HIF-1α 的表达,导致 HO-1 过表达、Fe2+ 生成过多、ROS 积累和 GPX 消耗以及铁变态反应。此外,使用Mito-TEMPO抑制线粒体还能下调HIF-1α/HO-1轴,减轻SHK诱导的铁变态反应。在体内,SHK 能有效抑制 OS 的生长,并具有良好的生物安全性,这证实了 SHK 诱导 OS 铁变态反应的分子机制。重要的是,我们证明了 HIF-1α 是由 MitoROS 间接调控的,而不是 SHK 直接与 HIF-1α 结合。我们的研究表明,SHK 是治疗 OS 的潜在候选药物,可能有助于确定 OS 的新型治疗靶点。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Phytomedicine
医学-药学
CiteScore
10.30
自引率
5.10%
发文量
670
审稿时长
91 days
期刊介绍:
Phytomedicine is a therapy-oriented journal that publishes innovative studies on the efficacy, safety, quality, and mechanisms of action of specified plant extracts, phytopharmaceuticals, and their isolated constituents. This includes clinical, pharmacological, pharmacokinetic, and toxicological studies of herbal medicinal products, preparations, and purified compounds with defined and consistent quality, ensuring reproducible pharmacological activity. Founded in 1994, Phytomedicine aims to focus and stimulate research in this field and establish internationally accepted scientific standards for pharmacological studies, proof of clinical efficacy, and safety of phytomedicines.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


