The key role of matrix stiffness in colorectal cancer immunotherapy: mechanisms and therapeutic strategies
IF 9.7
1区 医学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
Biochimica et biophysica acta. Reviews on cancer
Pub Date : 2024-10-15
DOI:10.1016/j.bbcan.2024.189198
引用次数: 0
Abstract
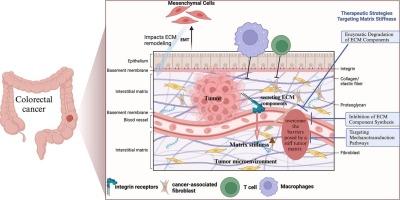
Increased matrix stiffness within the colorectal cancer (CRC) tumor microenvironment (TME) has emerged as a pivotal determinant of immunotherapy outcomes. This review discusses the role of aberrant extracellular matrix (ECM) deposition and cross-linking in augmenting matrix stiffness, a phenomenon that not only scaffolds the tumor architecture but also contributes to tumorigenicity and immunologic evasion. Herein, we critically appraise the influence of matrix stiffness on the immunotherapeutic landscape of CRC, focusing on its capacity to impede therapeutic efficacy by modulating immune cell infiltration, activation, and functional performance. The review explores the molecular dynamics whereby matrix stiffness prompts tumor evolution, highlighting the integral role of integrin signaling, cancer-associated fibroblasts (CAFs), and the process of epithelial-mesenchymal transition (EMT). We bring to the fore the paradoxical impact of an indurated ECM on immune effector cells, chiefly T cells and macrophages, which are indispensable for immune surveillance and the execution of immunotherapeutic strategies, yet are markedly restrained by a fibrotic matrix. Furthermore, we examine how matrix stiffness modulates immune checkpoint molecule expression, thereby exacerbating the immunosuppressive milieu within the TME and attenuating immunotherapeutic potency. Emergent therapeutic regimens targeting matrix stiffness—including matrix modulators, inhibitors of mechanotransduction signaling pathways, and advanced biomaterials that mimic the ECM—proffer novel modalities to potentiate immunotherapy responsiveness. By refining the ECM's biomechanical attributes, the mechanical barriers posed by the tumor stroma can be improved, facilitating robust immune cell penetration and activity, and thereby bolstering the tumor's susceptibility to immunotherapy. Ongoing clinical trials are evaluating these innovative treatments, particularly in combination with immunotherapies, with the aim of enhancing clinical outcomes for CRC patients afflicted by pronounced matrix stiffness.
基质硬度在结直肠癌免疫疗法中的关键作用:机制与治疗策略
结直肠癌(CRC)肿瘤微环境(TME)中基质硬度的增加已成为免疫疗法效果的关键决定因素。本综述讨论了细胞外基质(ECM)的异常沉积和交联在增强基质硬度中的作用,这种现象不仅为肿瘤结构提供了支架,还有助于肿瘤致病性和免疫逃避。在此,我们对基质僵化对 CRC 免疫治疗的影响进行了批判性评估,重点关注基质僵化通过调节免疫细胞浸润、活化和功能表现而阻碍疗效的能力。综述探讨了基质僵化促使肿瘤演化的分子动力学,强调了整合素信号、癌相关成纤维细胞(CAFs)和上皮-间质转化(EMT)过程的重要作用。我们揭示了硬化的 ECM 对免疫效应细胞(主要是 T 细胞和巨噬细胞)的矛盾影响,这些细胞对免疫监视和免疫治疗策略的实施不可或缺,但却受到纤维化基质的明显限制。此外,我们还研究了基质僵化如何调节免疫检查点分子的表达,从而加剧TME内的免疫抑制环境并削弱免疫治疗效力。针对基质硬度的新兴治疗方案--包括基质调节剂、机械传导信号通路抑制剂和模拟 ECM 的先进生物材料--提供了增强免疫疗法反应性的新模式。通过改进 ECM 的生物力学属性,可以改善肿瘤基质构成的机械屏障,促进免疫细胞的强力渗透和活性,从而提高肿瘤对免疫疗法的敏感性。目前正在进行的临床试验正在评估这些创新疗法,特别是与免疫疗法的结合,目的是提高受基质僵化困扰的癌症患者的临床疗效。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Biochimica et biophysica acta. Reviews on cancer
医学-生化与分子生物学
CiteScore
17.20
自引率
0.00%
发文量
138
审稿时长
33 days
期刊介绍:
Biochimica et Biophysica Acta (BBA) - Reviews on Cancer encompasses the entirety of cancer biology and biochemistry, emphasizing oncogenes and tumor suppressor genes, growth-related cell cycle control signaling, carcinogenesis mechanisms, cell transformation, immunologic control mechanisms, genetics of human (mammalian) cancer, control of cell proliferation, genetic and molecular control of organismic development, rational anti-tumor drug design. It publishes mini-reviews and full reviews.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


