Accumulation, biochemical responses and changes in the redox proteome promoted by Ag and Cd in the burrowing bivalve Scrobicularia plana
IF 4.1
2区 环境科学与生态学
Q1 MARINE & FRESHWATER BIOLOGY
引用次数: 0
Abstract
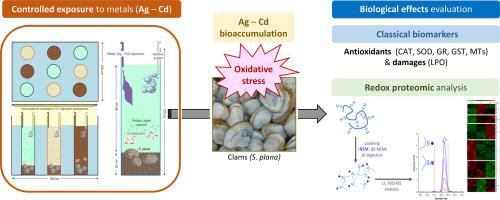
Silver (Ag) and cadmium (Cd) are non-essential metals that, as a result of natural processes and human activities, reach the aquatic environment where they interact with biota inducing potential toxic effects. To determine the biological effects of these metals on the endobenthic bivalve Scrobicularia plana, specimens were exposed to Ag and Cd at two concentrations, 5 and 50 μg∙L-1, for 7 days in a controlled microcosm system. The levels of the metals were measured in the seawater, sediments and clam tissues. The possible toxic biological effects of Ag and Cd were studied using a battery of biochemical biomarkers that are responsive to oxidative stress: superoxide dismutase (SOD), catalase (CAT), glutathione reductase (GR), glutathione-S-transferase (GST) activities, and metallothioneins (MTs) and lipid peroxidation (LPO) levels. Since both metals have been linked to oxidative stress, redox modifications to proteins were studied by differential isotopic labelling of the oxidised and reduced forms of cysteines (Cys). An accumulation of metals was observed in the digestive gland and gills following exposure, together with the activation of enzyme activities (SOD for the Cd exposure; SOD, CAT, GST, and GR for the Ag exposure). The MT and LPO levels (after individual exposure to Ag and Cd) increased, which suggests the existence of antioxidant and detoxification processes to mitigate the toxic oxidative effects of both metals. The redox proteomic analysis identified 771 Cys-containing peptides (out of 514 proteins), of which 195 and 226 changed after exposure to Ag and Cd, respectively. Bioinformatics analysis showed that exposure to metal affects relevant functional pathways and biological processes in S. plana, such as: “cellular respiration” (Ag), “metabolism of amino acids” and “synthesis and degradation of proteins” (Ag and Cd), “carbohydrate metabolism” and “oxidative stress” (Cd). The proteomic approach implemented here is a powerful complement to conventional biochemical biomarkers, since it evaluates changes at the protein level in a high-throughput unbiased manner, thus providing a general appraisal of the biological responses altered by exposure to the contaminants.
银和镉在穴居双壳贝Scrobicularia plana中的积累、生化反应和氧化还原蛋白质组的变化
银(Ag)和镉(Cd)是非基本金属,由于自然过程和人类活动,它们会进入水生环境,与生物群相互作用,产生潜在的毒性影响。为了确定这些金属对底栖双壳类动物 Scrobicularia plana 的生物影响,在一个受控的微宇宙系统中,将标本暴露于 5 和 50 μg∙L-1 两种浓度的银和镉中 7 天。测量了海水、沉积物和蛤蜊组织中的金属含量。利用一系列对氧化应激有反应的生化生物标志物:超氧化物歧化酶(SOD)、过氧化氢酶(CAT)、谷胱甘肽还原酶(GR)、谷胱甘肽-S-转移酶(GST)活性、金属硫蛋白(MTs)和脂质过氧化物(LPO)水平,研究了银和镉可能产生的毒性生物效应。由于这两种金属都与氧化应激有关,因此通过对半胱氨酸(Cys)的氧化和还原形式进行不同的同位素标记,对蛋白质的氧化还原修饰进行了研究。暴露后,在消化腺和鳃中观察到金属的积累,以及酶活性的激活(镉暴露为 SOD;银暴露为 SOD、CAT、GST 和 GR)。MT和LPO水平(分别暴露于Ag和Cd后)增加,这表明存在抗氧化和解毒过程,以减轻这两种金属的毒性氧化作用。氧化还原蛋白质组分析发现了 771 个含 Cys 的多肽(共有 514 个蛋白质),其中 195 个和 226 个在接触 Ag 和 Cd 后发生了变化。生物信息学分析表明,暴露于金属会影响S.plana的相关功能途径和生物过程,如"细胞呼吸"(银)、"氨基酸代谢 "和 "蛋白质合成与降解"(银和镉)、"碳水化合物代谢 "和 "氧化应激"(镉)。这里采用的蛋白质组学方法是对传统生化生物标志物的有力补充,因为它能以高通量、无偏见的方式评估蛋白质水平的变化,从而对暴露于污染物所改变的生物反应进行总体评估。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Aquatic Toxicology
环境科学-毒理学
CiteScore
7.10
自引率
4.40%
发文量
250
审稿时长
56 days
期刊介绍:
Aquatic Toxicology publishes significant contributions that increase the understanding of the impact of harmful substances (including natural and synthetic chemicals) on aquatic organisms and ecosystems.
Aquatic Toxicology considers both laboratory and field studies with a focus on marine/ freshwater environments. We strive to attract high quality original scientific papers, critical reviews and expert opinion papers in the following areas: Effects of harmful substances on molecular, cellular, sub-organismal, organismal, population, community, and ecosystem level; Toxic Mechanisms; Genetic disturbances, transgenerational effects, behavioral and adaptive responses; Impacts of harmful substances on structure, function of and services provided by aquatic ecosystems; Mixture toxicity assessment; Statistical approaches to predict exposure to and hazards of contaminants
The journal also considers manuscripts in other areas, such as the development of innovative concepts, approaches, and methodologies, which promote the wider application of toxicological datasets to the protection of aquatic environments and inform ecological risk assessments and decision making by relevant authorities.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


