Cuproptosis-based layer-by-layer silk fibroin nanoplatform-loaded PD-L1 siRNA combining photothermal and chemodynamic therapy against metastatic breast cancer
IF 8.7
1区 医学
Q1 ENGINEERING, BIOMEDICAL
引用次数: 0
Abstract
Cuproptosis is a newly identified form of copper-dependent cell death that differs from other known pathways. This discovery provides a new way to explore copper-based nanomaterial applications in cancer therapy. This study used a layer-by-layer self-assembling method to load Cu2-xS nanoparticle (NP) cores with the siRNA of the PD-L1 immune escape-related gene and wrap a silk fibroin (SF) shell to form a multifunctional copper-based SF nanoplatform, denoted as CuS-PEI-siRNA-SFNs. CuS-PEI-siRNA-SFNs induced cuproptosis and exerted an antitumor effect via multiple mechanisms, including photothermal therapy (PTT), chemodynamic therapy (CDT), and immune activation. The presence of significant dihydrolipoamide S-acetyltransferase (DLAT) oligomers in 4T1 cells treated with CuS-PEI-siRNA-SFNs indicated the triggering of cuproptosis. Furthermore, in vivo experimental results showed that CuS-PEI-siRNA-SFNs efficiently accumulated in the tumor tissues of 4T1 tumor-bearing mice inhibited primary tumor and lung metastasis, and displayed excellent biosafety and antitumor activity. This study demonstrated that the synergistic effect of cuproptosis, PTT, CDT, and immune activation showed promise for treating metastatic breast cancer.
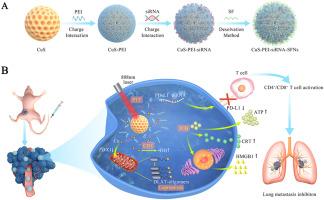
基于逐层丝纤维素纳米平台的 PD-L1 siRNA 光热疗法和化学动力疗法联合治疗转移性乳腺癌
铜中毒是一种新发现的铜依赖性细胞死亡形式,它不同于其他已知途径。这一发现为探索铜基纳米材料在癌症治疗中的应用提供了新途径。本研究采用逐层自组装的方法,在Cu2-xS纳米粒子(NP)内核中加入PD-L1免疫逃逸相关基因的siRNA,并包裹丝纤维蛋白(SF)外壳,形成多功能铜基SF纳米平台,即CuS-PEI-siRNA-SFNs。CuS-PEI-siRNA-SFNs通过光热疗法(PTT)、化学动力学疗法(CDT)和免疫激活等多种机制诱导杯突,并发挥抗肿瘤作用。用CuS-PEI-siRNA-SFNs处理的4T1细胞中存在大量二氢脂酰胺S-乙酰转移酶(DLAT)寡聚体,这表明它们引发了杯突变。此外,体内实验结果表明,CuS-PEI-siRNA-SFNs 能在 4T1 肿瘤小鼠的肿瘤组织中高效积累,抑制原发性肿瘤和肺转移,具有良好的生物安全性和抗肿瘤活性。这项研究表明,杯突、PTT、CDT 和免疫激活的协同作用有望治疗转移性乳腺癌。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Materials Today Bio
Multiple-
CiteScore
8.30
自引率
4.90%
发文量
303
审稿时长
30 days
期刊介绍:
Materials Today Bio is a multidisciplinary journal that specializes in the intersection between biology and materials science, chemistry, physics, engineering, and medicine. It covers various aspects such as the design and assembly of new structures, their interaction with biological systems, functionalization, bioimaging, therapies, and diagnostics in healthcare. The journal aims to showcase the most significant advancements and discoveries in this field. As part of the Materials Today family, Materials Today Bio provides rigorous peer review, quick decision-making, and high visibility for authors. It is indexed in Scopus, PubMed Central, Emerging Sources, Citation Index (ESCI), and Directory of Open Access Journals (DOAJ).
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


