Magnesiothermic reduction of beryllium fluoride: Reaction mechanism and kinetic study
IF 4.9
2区 工程技术
Q1 ENGINEERING, CHEMICAL
引用次数: 0
Abstract
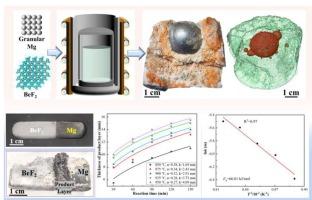
Beryllium (Be) is mainly produced by magnesiothermic reduction of beryllium fluoride (BeF2). This research aims to improve the extraction rate of Be by investigating the reaction mechanism and kinetics during the magnesiothermic reduction of BeF2. It was found that the solid product layer composed of MgF2 and Be metal produced during the magnesiothermic reduction process is the main reason hindering the further improvement of the reduction rate. Kinetic study on the magnesiothermic reduction of BeF2 shows that it was controlled by volume diffusion. An apparent activation energy of 66.01 kJ/mol was obtained for the magnesiothermic reduction in the temperature range of 850–950 °C. Aiming to extract Be from BeF2 with a high efficiency, granular-shaped Mg (particle size 0.2–5 mm) and BeF2 powder (particle size < 0.83 mm) were used as raw materials for magnesiothermic reduction at 900 °C for 30 min, protected using Ar atmosphere. This was followed by further heating to 1300 °C and holding for 10 min, and the highest extraction rate of Be was achieved at 90.1 wt% with the Be purity of 94.2 wt%.
氟化铍的镁热还原:反应机理和动力学研究
铍(Be)主要通过氟化铍(BeF2)的镁热还原生成。本研究旨在通过研究镁热还原 BeF2 过程中的反应机理和动力学,提高铍的提取率。研究发现,镁热还原过程中产生的由 MgF2 和金属 Be 组成的固体产物层是阻碍进一步提高还原率的主要原因。对 BeF2 镁热还原的动力学研究表明,它受体积扩散控制。在 850-950 °C 的温度范围内,镁热还原的表观活化能为 66.01 kJ/mol。为了从 BeF2 中高效提取 Be,以粒状 Mg(粒径 0.2-5 mm)和 BeF2 粉末(粒径 < 0.83 mm)为原料,在氩气保护下于 900 °C 下进行镁热还原 30 分钟。随后进一步加热至 1300 °C,保温 10 分钟,Be 的最高萃取率达到 90.1 wt%,Be 的纯度为 94.2 wt%。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Minerals Engineering
工程技术-工程:化工
CiteScore
8.70
自引率
18.80%
发文量
519
审稿时长
81 days
期刊介绍:
The purpose of the journal is to provide for the rapid publication of topical papers featuring the latest developments in the allied fields of mineral processing and extractive metallurgy. Its wide ranging coverage of research and practical (operating) topics includes physical separation methods, such as comminution, flotation concentration and dewatering, chemical methods such as bio-, hydro-, and electro-metallurgy, analytical techniques, process control, simulation and instrumentation, and mineralogical aspects of processing. Environmental issues, particularly those pertaining to sustainable development, will also be strongly covered.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


