Development of the novel advanced electrooxidation process for decolorization of recalcitrant dyes (Methylene Blue, Rhodamine B, Congo Red): Effect of operating factors
Q2 Environmental Science
引用次数: 0
Abstract
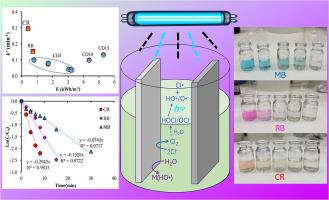
The research aims to develop an advanced electrooxidation process for decolorization of recalcitrant dye. To enhance the radical formation, a combination of UV irradiation and electrooxidation (UV/EO) is used. Methylene Blue (MB), Rhodamine B (RB) and Congo Red (CR) dyes were used as model compounds to represent recalcitrant dyes. The performance of UV/EO process was investigated under various operating factors (i.e., current density (CD), voltage, pH, NaCl, anodic material), and compared with chlorination, UV irradiation, and electrooxidation (EO) process. MB was the highest resistant dye to the UV/EO process. The UV/EO process exhibited a synergistic effect on decolorization, and it removed MB 1.35 time faster than the EO process. Based on indirect determination of •OH, the •OH formation in the EO process was 2.4 ×10−13 M, which lower than that in the UV/EO process by 4 times. The second-order rate constant for MB oxidation by HO• () was 3.31 × 109 M−1s−1. The pseudo 1st-order decolorization kinetic (k’) was independent with voltages, but directly depended on CD and NaCl concentrations. Lower pH enhanced the k’ value. The specific energy consumption was in a range of 0.408 – 5.303 kWh/m3, depending on the k’ value. The energy consumption decreased with higher the k’ value, except for increasing CD. The Boron Doped Diamond (BDD) was more effective in the decolorization rate than dimensional active anode (DSA) by 1.6 times. Treatment of industrial dye wastewater by the UV/EO process eliminated color intensity (ADMI), COD, and BOD5 by 80 %, 91 %, and 9 %, respectively.
开发新型高级电氧化工艺,用于脱除难分解染料(亚甲基蓝、罗丹明 B、刚果红)的颜色:操作因素的影响
这项研究旨在开发一种先进的电氧化工艺,用于对难降解染料进行脱色。为了增强自由基的形成,采用了紫外线照射和电氧化(UV/EO)相结合的方法。亚甲基蓝(MB)、罗丹明 B(RB)和刚果红(CR)染料被用作代表难分解染料的模型化合物。研究了 UV/EO 过程在各种操作因素(即电流密度 (CD)、电压、pH 值、NaCl、阳极材料)下的性能,并与氯化、UV 照射和电氧化 (EO) 过程进行了比较。MB 是对 UV/EO 工艺耐受性最高的染料。紫外线/电氧化工艺在脱色方面表现出协同效应,其去除甲基溴的速度比电氧化工艺快 1.35 倍。根据对 -OH 的间接测定,环氧乙烷工艺中 -OH 的生成量为 2.4 ×10-13 M,比紫外/环氧乙烷工艺低 4 倍。甲基溴被 HO- 氧化的二阶速率常数(kHO-,MB)为 3.31 × 109 M-1s-1。假一阶脱色动力学(k')与电压无关,但直接取决于 CD 和 NaCl 的浓度。pH 值越低,k'值越大。比能耗在 0.408 - 5.303 kWh/m3 之间,取决于 k' 值。k' 值越高,能耗越低,但 CD 值增加时除外。掺硼金刚石(BDD)的脱色率是二维活性阳极(DSA)的 1.6 倍。用紫外线/电离氧化工艺处理工业染料废水,色度(ADMI)、化学需氧量和生化需氧量(BOD5)的去除率分别为 80%、91% 和 9%。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Environmental Advances
Environmental Science-Environmental Science (miscellaneous)
CiteScore
7.30
自引率
0.00%
发文量
165
审稿时长
12 weeks
期刊介绍:
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


