PRKAA2-mediated mitophagy regulates oxygen consumption in yak renal tubular epithelial cells under chronic hypoxia
IF 4.4
2区 生物学
Q2 CELL BIOLOGY
引用次数: 0
Abstract
Hypoxic environments are significant factors in the induction of various kidney diseases and are closely associated with high oxygen consumption in the kidneys. Yaks live at high altitude for a long time, exhibit a unique ability to regulate kidney oxygen consumption, protecting them from hypoxia-induced damage. However, the mechanisms underlying the regulation of oxygen consumption in yak kidneys under hypoxic conditions remain unclear. To explore this hypoxia adaptation mechanism in yak kidneys, this study analyzed the oxygen consumption rate (OCR) of renal tubular epithelial cells (RTECs) under hypoxia. We found that the OCR and apoptosis rates of RTECs under chronic hypoxia (> 24 h) were lower than those under acute hypoxia (≤ 24 h). However, when oxygen consumption was promoted under chronic hypoxia, the apoptosis rate increased, indicating that reducing the cellular OCR is crucial for maintaining RTECs activity under hypoxia. High-throughput sequencing results showed that the mitophagy pathway is likely a key mechanism for inhibiting OCR of yak RTECs, with protein kinase AMP-activated catalytic subunit alpha 2 (PRKAA2) playing a significant role in this process. Further studies demonstrated that chronic hypoxia activates the mitophagy pathway, which inhibits oxidative phosphorylation (OXPHOS) while increasing glycolytic flux in yak RTECs. Conversely, when the mitophagy pathway was inhibited, there was an increase in the activity of OXPHOS enzymes and OCR. To further explore the role of PRKAA2 in the mitophagy pathway, we inhibited PRKAA2 expression under chronic hypoxia. Results showed that the downregulation of PRKAA2 decreased the expression of mitophagy-related proteins, such as p-FUNDC1/FUNDC1, LC3-II/LC3-I, BNIP3 and ULK1 while upregulating P62 expression. Additionally, there was an increase in the enzyme activities of Complex II, Complex IV, PDH, and SDH, which further promoted oxygen consumption in RTECs. These findings suggest that PRKAA2 mediated mitophagy under chronic hypoxia is crucial mechanism for reducing oxygen consumption in yak RTECs.
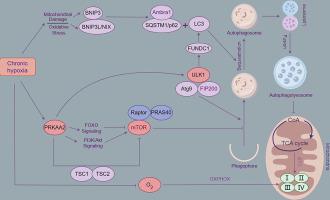
PRKAA2- 介导的有丝分裂调节慢性缺氧条件下牦牛肾小管上皮细胞的耗氧量
缺氧环境是诱发各种肾脏疾病的重要因素,与肾脏的高耗氧量密切相关。牦牛长期生活在高海拔地区,具有独特的调节肾脏耗氧量的能力,可保护肾脏免受缺氧引起的损伤。然而,缺氧条件下牦牛肾脏耗氧量的调节机制仍不清楚。为了探索牦牛肾脏的这种缺氧适应机制,本研究分析了缺氧条件下肾小管上皮细胞(RTECs)的耗氧量(OCR)。我们发现,慢性缺氧(24小时)条件下肾小管上皮细胞的耗氧率和凋亡率均低于急性缺氧(≤24小时)条件下的耗氧率和凋亡率。然而,在慢性缺氧条件下,当促进氧消耗时,细胞凋亡率增加,这表明降低细胞OCR对维持RTECs在缺氧条件下的活性至关重要。高通量测序结果显示,有丝分裂途径可能是抑制牦牛RTECs OCR的关键机制,而蛋白激酶AMP激活催化亚基α2(PRKAA2)在这一过程中发挥着重要作用。进一步的研究表明,慢性缺氧会激活有丝分裂途径,从而抑制氧化磷酸化(OXPHOS),同时增加牦牛RTEC的糖酵解通量。相反,当抑制有丝分裂途径时,OXPHOS 酶和 OCR 的活性会增加。为了进一步探讨PRKAA2在有丝分裂途径中的作用,我们在慢性缺氧条件下抑制了PRKAA2的表达。结果显示,下调PRKAA2会降低有丝分裂相关蛋白的表达,如p-FUNDC1/FUNDC1、LC3-II/LC3-I、BNIP3和ULK1,同时上调P62的表达。此外,复合体 II、复合体 IV、PDH 和 SDH 的酶活性也有所增加,这进一步促进了 RTECs 的耗氧量。这些发现表明,PRKAA2在慢性缺氧条件下介导的有丝分裂是降低牦牛RTEC耗氧量的关键机制。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Cellular signalling
生物-细胞生物学
CiteScore
8.40
自引率
0.00%
发文量
250
审稿时长
27 days
期刊介绍:
Cellular Signalling publishes original research describing fundamental and clinical findings on the mechanisms, actions and structural components of cellular signalling systems in vitro and in vivo.
Cellular Signalling aims at full length research papers defining signalling systems ranging from microorganisms to cells, tissues and higher organisms.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


