Separation of molybdenum and tungsten using selective adsorption with zirconium based metal organic framework
IF 5.5
3区 工程技术
Q1 ENGINEERING, CHEMICAL
Journal of the Taiwan Institute of Chemical Engineers
Pub Date : 2024-10-18
DOI:10.1016/j.jtice.2024.105802
引用次数: 0
Abstract
Background
The separation of tungsten and molybdenum has always been a significant challenge. Metal organic frameworks (MOFs) has potential to solve the problem. In this study, the adsorption and separation performance of UiO-66 for tungsten and molybdenum and adsorption mechanism were investigated.
Methods
UiO-66 was synthesized by solvothermal method. The physical and chemical properties of UiO-66 and adsorption mechanism were characterized and analyzed by XRD, SEM-EDS, FT-IR, Zeta-potential, XPS and thermodynamic analysis, the adsorption and separation performance of Mo/W using UiO-66 were evaluated by ICP-OES.
Significant findings
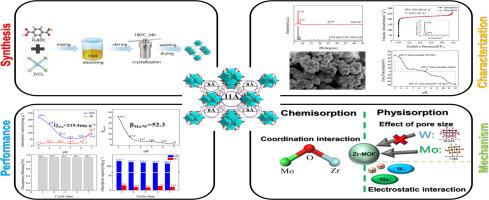
UiO-66 with uniform pore size could be obtained under the conditions of crystallization for 24 h at 180 °C. This study revealed superior Mo/W separation performance under acidic conditions, where the adsorption capacity for Mo (QMo) of UiO-66 was 219.4 mg⋅g−1 and the highest separation factor (βMo/W) was 52.3. It was found that the mechanism involved the effect of pore size, electrostatic attraction and the metal point Zr had stronger affinity for Mo. This material was expected to serve as an eco-friendly and reusable adsorbent for separation of tungsten and molybdenum in resource recovery, aiming for high-quality regeneration of both metals.
利用锆基金属有机框架的选择性吸附分离钼和钨
背景钨和钼的分离一直是一项重大挑战。金属有机框架(MOFs)具有解决这一问题的潜力。本研究考察了 UiO-66 对钨和钼的吸附分离性能及吸附机理。通过 XRD、SEM-EDS、FT-IR、Zeta 电位、XPS 和热力学分析对 UiO-66 的理化性质和吸附机理进行了表征和分析,并通过 ICP-OES 评估了 UiO-66 对 Mo/W 的吸附和分离性能。该研究揭示了 UiO-66 在酸性条件下卓越的 Mo/W 分离性能,其对 Mo 的吸附容量(QMo)为 219.4 mg-g-1,最高分离因子(βMo/W)为 52.3。研究发现,其机理涉及孔隙大小、静电吸引和金属点 Zr 对 Mo 更强亲和力的影响。该材料有望成为一种环保且可重复使用的吸附剂,用于在资源回收中分离钨和钼,从而实现这两种金属的高质量再生。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
9.10
自引率
14.00%
发文量
362
审稿时长
35 days
期刊介绍:
Journal of the Taiwan Institute of Chemical Engineers (formerly known as Journal of the Chinese Institute of Chemical Engineers) publishes original works, from fundamental principles to practical applications, in the broad field of chemical engineering with special focus on three aspects: Chemical and Biomolecular Science and Technology, Energy and Environmental Science and Technology, and Materials Science and Technology. Authors should choose for their manuscript an appropriate aspect section and a few related classifications when submitting to the journal online.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


