Comprehensive in silico characterization of Arabidopsis thaliana RecQl helicases through structure prediction and molecular dynamics simulations
IF 3.6
3区 综合性期刊
Q1 MULTIDISCIPLINARY SCIENCES
引用次数: 0
Abstract
Helicases are ubiquitous enzymes with specific functions that contribute to almost all nucleic acid metabolic processes. The RecQ helicase family is essential for integrity in all organisms through DNA replication, repair, and recombination. This study investigated five RecQ-like helicases in Arabidopsis thaliana (AtRecQl) that exhibit diverse structural and physiochemical attributes and functions. Cis-regulatory element analysis identified stress, hormone, cell cycle, and development-responsive modules involved in various events in plant growth and development. Gene ontology analysis revealed that the five AtRecQl were associated with various cellular components, molecular functions, and biological processes. Protein-protein interaction analysis also implicated some in various abiotic stress processes. Structural analysis and molecular dynamics (MD) simulations were performed to examine conformational stability through root means square deviation and radius of gyration, showing stable AtRecQl protein structures. Free energy landscape analysis validated thermodynamically stable structures throughout the MD simulation. Principle component analysis and probability density functions from MD simulations provided satisfactory structural variational data for the complexes and limited coordinate movements. These insights might greatly benefit future studies.
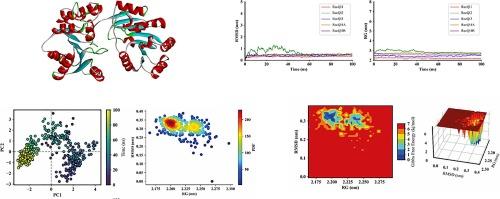
通过结构预测和分子动力学模拟对拟南芥 RecQl 螺旋酶进行全面的硅学鉴定
螺旋酶是一种无处不在的酶,具有特殊功能,几乎参与了所有核酸代谢过程。RecQ 螺旋酶家族通过 DNA 复制、修复和重组对所有生物的完整性至关重要。本研究调查了拟南芥(AtRecQl)中的五种类似 RecQ 的螺旋酶,它们表现出不同的结构和理化属性及功能。顺式调控元件分析确定了参与植物生长和发育过程中各种事件的胁迫、激素、细胞周期和发育响应模块。基因本体分析表明,五种 AtRecQl 与各种细胞成分、分子功能和生物过程有关。蛋白质-蛋白质相互作用分析还表明,其中一些与各种非生物胁迫过程有关。通过均方根偏差和回转半径进行结构分析和分子动力学(MD)模拟,研究了构象稳定性,结果显示 AtRecQl 蛋白结构稳定。自由能谱分析验证了整个 MD 模拟过程中的热力学稳定结构。来自 MD 模拟的主成分分析和概率密度函数为复合物和有限的坐标移动提供了令人满意的结构变异数据。这些见解对今后的研究大有裨益。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of King Saud University - Science
Multidisciplinary-Multidisciplinary
CiteScore
7.20
自引率
2.60%
发文量
642
审稿时长
49 days
期刊介绍:
Journal of King Saud University – Science is an official refereed publication of King Saud University and the publishing services is provided by Elsevier. It publishes peer-reviewed research articles in the fields of physics, astronomy, mathematics, statistics, chemistry, biochemistry, earth sciences, life and environmental sciences on the basis of scientific originality and interdisciplinary interest. It is devoted primarily to research papers but short communications, reviews and book reviews are also included. The editorial board and associated editors, composed of prominent scientists from around the world, are representative of the disciplines covered by the journal.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


