Electrochemically driven nonheme iron complex-catalyzed oxidation reactions using water as an oxygen source
IF 6.5
1区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
High-valent metal-oxo species have been invoked as key intermediates in enzymatic and biomimetic oxidation reactions. The generation of high-valent metal-oxo species using water (H2O) as an oxygen source represents one of the most environmentally friendly approaches in developing biologically inspired oxidation catalysis. Herein, we report the electrochemical oxidation of benzylic C−H bonds and alcohols utilizing a mononuclear nonheme iron(III)-monoamidate complex [FeIII(dpaq)(H2O)]2+ (dpaq = 2-[bis(pyridin-2-ylmethyl)]amino-N-quinolin-8-yl-acetamidate) as a catalyst and H2O as an oxygen source. Selective benzylic C−H bond oxidation of alkanes to ketones was achieved in 43–85 % yields, and primary and secondary alcohols were converted to the corresponding aldehydes and ketones, respectively, in 46–95 % yields. The generation of an iron(V)-oxo species [FeV(O)(dpaq)]2+ from proton-coupled electron-transfer (PCET) oxidation of the iron(III) aqua complex [FeIII(dpaq)(H2O)]2+ was evidenced by cyclic voltammetry analysis; the iron(V)-oxo species [FeV(O)(dpaq)]2+ was recently detected using transient absorption spectroscopy in water oxidation reactions. Mechanistic studies revealed that electrochemical oxidation of alcohols catalyzed by FeIII(dpaq) is a two-electron oxidation process, hydrogen-atom transfer (HAT) from the α-C−H bond of alcohols by iron(V)-oxo species is the rate-determining step, and there is a remarkable charge transfer from the highly electrophilic iron(V)-oxo species to the alcohols in the HAT step. This research paves a significant groundwork aimed at developing electrochemically driven biomimetic asymmetric oxidation reactions catalyzed by nonheme metal complexes supported by chiral ligands.
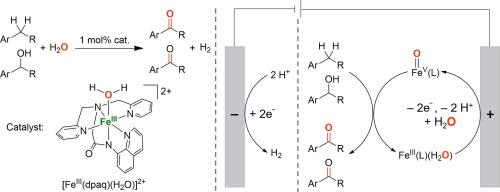
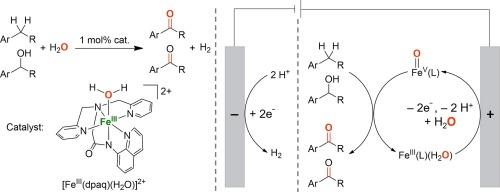
以水为氧源的电化学驱动非血红素铁络合物催化氧化反应
高价金属氧物种被认为是酶促和仿生氧化反应的关键中间产物。利用水(H2O)作为氧源生成高价金属氧物种是开发生物氧化催化反应的最环保方法之一。在此,我们报告了利用单核非血红素铁(III)-单酰胺复合物 [FeIII(dpaq)(H2O)]2+ (dpaq = 2-[双(吡啶-2-基甲基)]氨基-N-喹啉-8-基乙酰胺酯)作为催化剂和 H2O 作为氧源对苄基 C-H 键和醇进行电化学氧化的情况。烷烃到酮的选择性苄基 C-H 键氧化反应的收率为 43-85%,伯醇和仲醇分别转化为相应的醛和酮的收率为 46-95%。通过循环伏安分析,证明了铁(III)水络合物[FeIII(dpaq)(H2O)]2+在质子耦合电子转移(PCET)氧化过程中生成了铁(V)-氧化物[FeV(O)(dpaq)]2+;最近,在水氧化反应中使用瞬态吸收光谱检测到了铁(V)-氧化物[FeV(O)(dpaq)]2+。机理研究发现,FeIII(dpaq)催化的醇的电化学氧化是一个双电子氧化过程,铁(V)-氧化物从醇的α-C-H键转移氢原子(HAT)是决定速率的步骤,在HAT步骤中,高亲电性的铁(V)-氧化物与醇之间存在显著的电荷转移。这项研究为开发由手性配体支持的非血红素金属配合物催化的电化学驱动的仿生物不对称氧化反应奠定了重要基础。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Catalysis
工程技术-工程:化工
CiteScore
12.30
自引率
5.50%
发文量
447
审稿时长
31 days
期刊介绍:
The Journal of Catalysis publishes scholarly articles on both heterogeneous and homogeneous catalysis, covering a wide range of chemical transformations. These include various types of catalysis, such as those mediated by photons, plasmons, and electrons. The focus of the studies is to understand the relationship between catalytic function and the underlying chemical properties of surfaces and metal complexes.
The articles in the journal offer innovative concepts and explore the synthesis and kinetics of inorganic solids and homogeneous complexes. Furthermore, they discuss spectroscopic techniques for characterizing catalysts, investigate the interaction of probes and reacting species with catalysts, and employ theoretical methods.
The research presented in the journal should have direct relevance to the field of catalytic processes, addressing either fundamental aspects or applications of catalysis.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


