PLD3 and PLD4 synthesize S,S-BMP, a key phospholipid enabling lipid degradation in lysosomes
IF 45.5
1区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
Bis(monoacylglycero)phosphate (BMP) is an abundant lysosomal phospholipid required for degradation of lipids, particularly gangliosides. Alterations in BMP levels are associated with neurodegenerative diseases. Unlike typical glycerophospholipids, lysosomal BMP has two chiral glycerol carbons in the S (rather than the R) stereo-conformation, protecting it from lysosomal degradation. How this unusual and yet crucial S,S-stereochemistry is achieved is unknown. Here, we report that phospholipases D3 and D4 (PLD3 and PLD4) synthesize lysosomal S,S-BMP, with either enzyme catalyzing the critical glycerol stereo-inversion reaction in vitro. Deletion of PLD3 or PLD4 markedly reduced BMP levels in cells or in murine tissues where either enzyme is highly expressed (brain for PLD3; spleen for PLD4), leading to gangliosidosis and lysosomal abnormalities. PLD3 mutants associated with neurodegenerative diseases, including risk of Alzheimer’s disease, diminished PLD3 catalytic activity. We conclude that PLD3/4 enzymes synthesize lysosomal S,S-BMP, a crucial lipid for maintaining brain health.
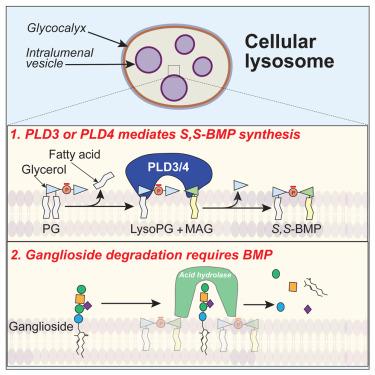
PLD3 和 PLD4 合成 S,S-BMP,这是一种能在溶酶体中实现脂质降解的关键磷脂
双(单酰基甘油)磷酸酯(BMP)是一种丰富的溶酶体磷脂,用于降解脂质,尤其是神经节苷脂。BMP 水平的变化与神经退行性疾病有关。与典型的甘油磷脂不同,溶酶体 BMP 有两个手性甘油碳位于 S(而不是 R)立体构象中,从而保护它不被溶酶体降解。这种不同寻常但又至关重要的 S,S 立体化学是如何实现的,目前尚不清楚。在这里,我们报告了磷脂酶 D3 和 D4(PLD3 和 PLD4)合成溶酶体 S,S-BMP,其中任何一种酶都能在体外催化关键的甘油立体转化反应。缺失 PLD3 或 PLD4 会明显降低细胞或小鼠组织中的 BMP 水平,而这两种酶在细胞或小鼠组织中都有很高的表达量(PLD3 在脑;PLD4 在脾),从而导致神经节苷脂病和溶酶体异常。与神经退行性疾病(包括阿尔茨海默病风险)相关的 PLD3 突变体会降低 PLD3 的催化活性。我们的结论是,PLD3/4 酶合成溶酶体 S,S-BMP,这是一种维持大脑健康的重要脂质。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Cell
生物-生化与分子生物学
CiteScore
110.00
自引率
0.80%
发文量
396
审稿时长
2 months
期刊介绍:
Cells is an international, peer-reviewed, open access journal that focuses on cell biology, molecular biology, and biophysics. It is affiliated with several societies, including the Spanish Society for Biochemistry and Molecular Biology (SEBBM), Nordic Autophagy Society (NAS), Spanish Society of Hematology and Hemotherapy (SEHH), and Society for Regenerative Medicine (Russian Federation) (RPO).
The journal publishes research findings of significant importance in various areas of experimental biology, such as cell biology, molecular biology, neuroscience, immunology, virology, microbiology, cancer, human genetics, systems biology, signaling, and disease mechanisms and therapeutics. The primary criterion for considering papers is whether the results contribute to significant conceptual advances or raise thought-provoking questions and hypotheses related to interesting and important biological inquiries.
In addition to primary research articles presented in four formats, Cells also features review and opinion articles in its "leading edge" section, discussing recent research advancements and topics of interest to its wide readership.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


