Insulin-Like Growth Factor Binding Protein 2 Drives Neurodegeneration in Parkinson's Disease: Insights From In Vivo and In Vitro Studies
Abstract
Aims
Insulin-like growth factor binding protein 2 (IGFBP2) is implicated in various neurodegenerative diseases. However, its role in Parkinson's disease (PD) is unclear.
Methods
PD rat model was established by 6-OHDA injection. After 3 weeks, mRNA-seq was conducted. Rats received rIGFBP2 via intra-MFB injection 6 h prior to 6-OHDA infusion, and the effect of IGFBP2 in PD rats was investigated by western blotting, IHC, specific kits, JC-1 staining, and TUNEL analysis. In vitro, PC12 cells were treated with 6-OHDA, and CCK-8, specific kits, Hoechst 33258 staining, Western blotting, and JC-1 staining were performed to assess the IGFBP2's role.
Results
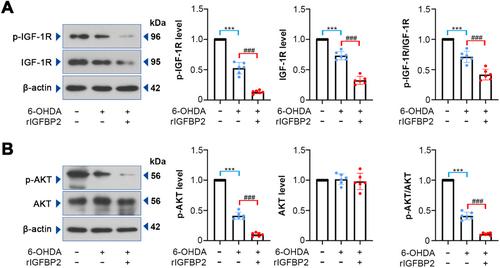
mRNA-seq revealed DEGs in PD, with attention to downregulated IGFBP2. rIGFBP2 treatment aggravated neurobehavioral deficits, decreased TH expression, Ψm, ATP level and SOD, GSH-Px activities but increased α-synuclein, ROS, MDA, mitochondrial cytochrome c contents, cell apoptosis in 6-OHDA-lesioned rats, which might be mediated through inactivating IGF-1R/AKT pathway. In 6-OHDA-treated PC12 cells, rIGFBP2 aggravated cell injury, demonstrated by decreased cell viability and increased apoptosis, oxidative stress, and mitochondrial dysfunction. Co-treatment with rIGFBP2 and rIGF-1 partially reversed the effect of rIGFBP2 on cell damage.
Conclusion
IGFBP2 exacerbates neurodegeneration in PD through increasing oxidative stress, mitochondrial dysfunction, and apoptosis via inhibiting IGF-1R/AKT pathway.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


