The Mechanism of Deprotonation of the Amino Group of Glutamate upon Binding to N-Acetylglutamate Synthase
IF 0.5
Q4 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
Gcn5-related N-acetyltransferases catalyze the transfer of an acetyl group to a primary amino group of a wide class of substrates. Deprotonation of the amino group upon binding to the enzyme is necessary to activate the nucleophilic attack on the substrate. The process of binding of glutamate to N-acetylglutamate synthase is considered using the methods of molecular modeling and quantum chemistry. It is shown that deprotonation of the primary amino group of glutamate occurs upon its incorporation into the active site of the enzyme with the participation of the side chain of the aspartate residue.
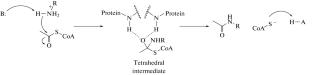
谷氨酸氨基与 N-乙酰谷氨酸合成酶结合后的去质子化机制
Gcn5 相关 N-乙酰转移酶可催化乙酰基向多种底物的伯氨基转移。氨基与酶结合后必须进行去质子化,才能激活对底物的亲核攻击。本研究采用分子建模和量子化学的方法研究了谷氨酸与 N-乙酰谷氨酸合成酶的结合过程。研究表明,谷氨酸的伯氨基在天冬氨酸残基侧链的参与下进入酶的活性位点后会发生去质子化作用。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Moscow University Chemistry Bulletin
CHEMISTRY, MULTIDISCIPLINARY-
CiteScore
1.30
自引率
14.30%
发文量
38
期刊介绍:
Moscow University Chemistry Bulletin is a journal that publishes review articles, original research articles, and short communications on various areas of basic and applied research in chemistry, including medical chemistry and pharmacology.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


