Interaction of Copper Clusters with Dioxidine
IF 0.5
Q4 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
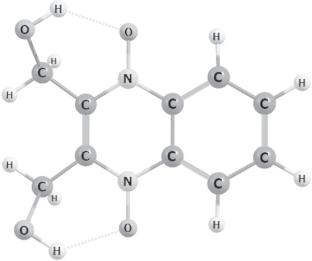
The configurations of small copper clusters (Cu2, Cu3, Cu13) and their complexes including a complex containing a copper atom with dioxidine (Dx) are calculated by density functional theory modeling with B3LYP5 parametrization. The trends of the changes in the geometry configuration and metal cluster–dioxidine ligand interaction energy depending on the size of the metal cluster are assessed. The dissociation energy of the complexes increases with the metal cluster size but the maximum value (55.1 kcal/mol) is implemented for a Cu3–Dx complex. There is coordination of the metal atom to one or two oxygen atoms of the dioxidine molecule for all the complexes.
铜簇与二氧六环的相互作用
采用 B3LYP5 参数化的密度泛函理论模型计算了小型铜簇(Cu2、Cu3、Cu13)及其配合物(包括含有一个铜原子的配合物与二氧杂环丁(Dx))的构型。评估了金属簇的几何构型和金属簇-二恶烷配体相互作用能随金属簇大小而变化的趋势。配合物的解离能随金属簇的大小而增加,但 Cu3-Dx 配合物的解离能达到了最大值(55.1 kcal/mol)。所有络合物的金属原子都与二噁烷分子的一个或两个氧原子配位。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Moscow University Chemistry Bulletin
CHEMISTRY, MULTIDISCIPLINARY-
CiteScore
1.30
自引率
14.30%
发文量
38
期刊介绍:
Moscow University Chemistry Bulletin is a journal that publishes review articles, original research articles, and short communications on various areas of basic and applied research in chemistry, including medical chemistry and pharmacology.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


