Influence of 5-fluorination on the structure and glycosidic bond stability of the protonated canonical DNA and RNA cytidine nucleosides: Oh, what a difference the 2′-hydroxy substituent makes?
IF 1.7
3区 化学
Q3 PHYSICS, ATOMIC, MOLECULAR & CHEMICAL
引用次数: 0
Abstract
Fluorinated nucleosides are well-established as anticancer and antiviral medications. As with many pharmaceuticals, the effects of fluorine modifications are often only partially understood. In this work, the effects of 5-fluorination of the cytosine nucleobase on the structures and glycosidic bond stabilities of the protonated canonical DNA and RNA cytidine nucleosides (dCyd and Cyd) are examined. Infrared multiple photon dissociation action spectroscopy experiments and electronic structure calculations are employed to probe the structural influences of 5-fluorination. Spectral signatures in the IR fingerprint and hydrogen-stretching regions indicate that 5-fluorination heavily directs for and solely produces O2 protonation para to the 5-fluoro substituent. This differs from the canonical cytidine nucleosides where roughly equal populations of O2 and N3 protonated structures were observed. Energy-resolved collision-induced dissociation experiments combined with survival yield analyses are performed to probe the influence of 5-fluorination on glycosidic bond stability. Trends in the energy-dependence of the survival yield curves indicate that 5-fluorination weakens the glycosidic bond, and that the influence of 5-fluorination on glycosidic bond stability is much greater for dCyd than Cyd. Theory also finds that 5-fluorination weakens the glycosidic bond but predicts that the influence of 5-fluorination on N-glycosidic bond stability is roughly the same for dCyd and Cyd. Oh, what a difference the 2′-hydroxy substituent makes?
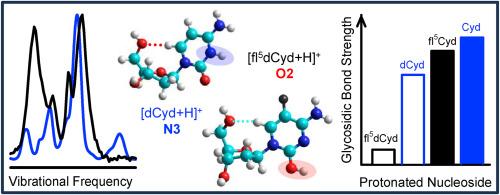
5-氟化对质子化 DNA 和 RNA 胞苷核苷结构和糖苷键稳定性的影响:哦,2′-羟基取代基带来了多大的不同?
氟化核苷是公认的抗癌和抗病毒药物。与许多药物一样,人们对氟修饰的影响往往只有部分了解。在这项研究中,我们研究了胞嘧啶核碱基的 5-氟化对质子化 DNA 和 RNA 胞嘧啶核苷(dCyd 和 Cyd)的结构和糖苷键稳定性的影响。红外多光子解离作用光谱实验和电子结构计算被用来探究 5-氟化对结构的影响。红外指纹区和氢伸展区的光谱特征表明,5-氟化作用对 5-氟取代基的 O2 质子化具有很大的指导作用,而且只产生 O2 质子化。这与典型的胞嘧啶核苷不同,在后者中观察到的 O2 和 N3 质子化结构大致相同。能量分辨碰撞诱导解离实验与存活率分析相结合,探究了 5-氟化对糖苷键稳定性的影响。存活率曲线的能量依赖性趋势表明,5-氟化会削弱糖苷键,而且 5-氟化对糖苷键稳定性的影响对 dCyd 比对 Cyd 要大得多。理论也发现 5-氟化会削弱糖苷键,但预测 5-氟化对 N-糖苷键稳定性的影响对 dCyd 和 Cyd 大致相同。哦,2′-羟基取代基带来了多大的不同?
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
3.60
自引率
5.60%
发文量
145
审稿时长
71 days
期刊介绍:
The journal invites papers that advance the field of mass spectrometry by exploring fundamental aspects of ion processes using both the experimental and theoretical approaches, developing new instrumentation and experimental strategies for chemical analysis using mass spectrometry, developing new computational strategies for data interpretation and integration, reporting new applications of mass spectrometry and hyphenated techniques in biology, chemistry, geology, and physics.
Papers, in which standard mass spectrometry techniques are used for analysis will not be considered.
IJMS publishes full-length articles, short communications, reviews, and feature articles including young scientist features.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


