Multi-omics analysis reveal the fall armyworm Spodoptera frugiperda tolerate high temperature by mediating chitin-related genes
IF 3.2
2区 农林科学
Q2 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
Climate change facilitates the rapid invasion of agricultural pests, threatening global food security. The fall armyworm Spodoptera frugiperda is a highly polyphagous migratory pest tolerant to high temperatures, allowing its proliferation in harsh thermal environments. We aimed to demonstrate mechanisms of its high-temperature tolerance, particularly transcriptional and metabolic regulation, which are poorly understood. To achieve the aim, we examined the impact and mechanism of heat events on S. frugiperda by using multiple approaches: ecological measurements, transcriptomics, metabolomics, RNAi, and CRISPR/Cas9 technology. We observed that several physiological indices (larval survival rate, larval period, pupation rate, pupal weight, eclosion rate, and average fecundity) decreased as the temperature increased, with the 32 °C treatment displaying a significant difference from the control group at 26 °C. Significantly upregulated expression of genes encoding endochitinase and chitin deacetylase was observed in the chitin-binding, extracellular region, and carbohydrate metabolic process GO terms of hemolymph, fat body, and brain, exhibiting a tissue-specific pattern. Significantly enriched pathways (e.g., cutin, suberin, and wax biosynthesis; oxidative phosphorylation and cofactor biosynthesis; diverse amino acid biosynthesis and degradation; carbon metabolism; and energy metabolism), all of which are essential for S. frugiperda larvae to tolerate temperature, were found in metabolites that were expressed differently. Successful RNA interference targeting of the three chitin-related genes reduced gene expression levels and larval survival rate. Knockout of the endochitinase gene by using the CRISPR/Cas9 system significantly reduced the relative gene expression and increased sensitivity to high-temperature exposure. On the basis of our findings, theoretical foundations for understanding the high-temperature tolerance of S. frugiperda populations and latent genetic control strategies were established.
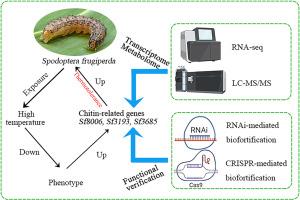
多组学分析揭示秋军虫通过介导几丁质相关基因耐高温
气候变化助长了农业害虫的快速入侵,威胁着全球粮食安全。秋虫(Spodoptera frugiperda)是一种高度多食性迁飞害虫,耐高温,可在恶劣的热环境中繁殖。我们的目标是证明其耐高温的机制,特别是转录和代谢调控机制,目前对这些机制还知之甚少。为了实现这一目标,我们采用多种方法研究了高温事件对 S. frugiperda 的影响和机制:生态测量、转录组学、代谢组学、RNAi 和 CRISPR/Cas9 技术。我们观察到,一些生理指标(幼虫存活率、幼虫期、化蛹率、蛹重、羽化率和平均受精率)随着温度的升高而降低,32 °C处理组与26 °C对照组有显著差异。内几丁质酶和几丁质脱乙酰酶的编码基因在几丁质结合、细胞外区域和碳水化合物代谢过程GO方面的表达显著上调,表现出组织特异性。在表达不同的代谢物中发现了显著富集的途径(如角质素、单宁和蜡的生物合成;氧化磷酸化和辅助因子的生物合成;多种氨基酸的生物合成和降解;碳代谢;以及能量代谢),所有这些途径都是弗氏幼虫耐受温度所必需的。成功地对这三个几丁质相关基因进行 RNA 干扰,降低了基因表达水平和幼虫存活率。利用CRISPR/Cas9系统敲除内几丁质酶基因可显著降低基因的相对表达量,提高对高温暴露的敏感性。根据我们的研究结果,建立了了解节肢动物种群耐高温性和潜在基因控制策略的理论基础。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
7.40
自引率
5.30%
发文量
105
审稿时长
40 days
期刊介绍:
This international journal publishes original contributions and mini-reviews in the fields of insect biochemistry and insect molecular biology. Main areas of interest are neurochemistry, hormone and pheromone biochemistry, enzymes and metabolism, hormone action and gene regulation, gene characterization and structure, pharmacology, immunology and cell and tissue culture. Papers on the biochemistry and molecular biology of other groups of arthropods are published if of general interest to the readership. Technique papers will be considered for publication if they significantly advance the field of insect biochemistry and molecular biology in the opinion of the Editors and Editorial Board.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


