In situ photocrosslinkable hydrogel treats radiation-induced skin injury by ROS elimination and inflammation regulation
IF 12.8
1区 医学
Q1 ENGINEERING, BIOMEDICAL
引用次数: 0
Abstract
The clinical management of radiation-induced skin injury (RSI) poses a significant challenge, primarily due to the acute damage caused by an overabundance of reactive oxygen species (ROS) and the ongoing inflammatory microenvironment. Here, we designed a dual-network hydrogel composed of 5 % (w/v) Pluronic F127 diacrylate and 2 % (w/v) hyaluronic acid methacryloyl, termed the FH hydrogel. To confer antioxidant and anti-inflammation properties to the hydrogel, we incorporated PVP-modified Prussian blue nanoparticles (PPBs) and resveratrol (Res) to form PHF@Res hydrogels. PHF@Res hydrogels not only exhibited multiple free radical scavenging activities (DPPH, ABTS), but also displayed multiple enzyme-like activities (POD-, catalase). Meanwhile, PHF@Res-2 hydrogels significantly suppressed intracellular ROS and promoted the migration of fibroblasts in a high-oxidative stress environment. Moreover, in the RSI mouse model, the PHF@Res-2 hydrogel regulated inflammatory factors and collagen deposition, significantly reduced epithelial hyperplasia, promoted limb regeneration and neovascularization, and accelerated wound healing, outperforming the commercial antiradiation formulation, Kangfuxin. The PHF@Res-2 hydrogel dressing shows great potential in accelerating wound healing in RSI, offering tremendous promise for clinical wound management and regeneration.
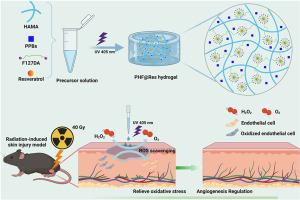
原位光交联水凝胶通过消除 ROS 和调节炎症治疗辐射引起的皮肤损伤
辐射诱发皮肤损伤(RSI)的临床治疗是一项重大挑战,这主要是由于过量的活性氧(ROS)和持续的炎症微环境造成的急性损伤。在这里,我们设计了一种双网络水凝胶,由 5% (w/v) Pluronic F127 二丙烯酸酯和 2% (w/v) 甲基丙烯酰透明质酸组成,称为 FH 水凝胶。为了赋予水凝胶抗氧化和抗炎特性,我们加入了 PVP 改性普鲁士蓝纳米粒子(PPBs)和白藜芦醇(Res),形成 PHF@Res 水凝胶。PHF@Res 水凝胶不仅具有多种自由基清除活性(DPPH、ABTS),还具有多种类酶活性(POD、过氧化氢酶)。同时,PHF@Res-2 水凝胶还能显著抑制细胞内的 ROS,促进成纤维细胞在高氧化应激环境下的迁移。此外,在 RSI 小鼠模型中,PHF@Res-2 水凝胶能调节炎症因子和胶原沉积,明显减少上皮增生,促进肢体再生和血管新生,加速伤口愈合,其效果优于商业抗辐射制剂康复新。PHF@Res-2 水凝胶敷料在加速 RSI 伤口愈合方面显示出巨大潜力,为临床伤口管理和再生带来了巨大希望。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Biomaterials
工程技术-材料科学:生物材料
CiteScore
26.00
自引率
2.90%
发文量
565
审稿时长
46 days
期刊介绍:
Biomaterials is an international journal covering the science and clinical application of biomaterials. A biomaterial is now defined as a substance that has been engineered to take a form which, alone or as part of a complex system, is used to direct, by control of interactions with components of living systems, the course of any therapeutic or diagnostic procedure. It is the aim of the journal to provide a peer-reviewed forum for the publication of original papers and authoritative review and opinion papers dealing with the most important issues facing the use of biomaterials in clinical practice. The scope of the journal covers the wide range of physical, biological and chemical sciences that underpin the design of biomaterials and the clinical disciplines in which they are used. These sciences include polymer synthesis and characterization, drug and gene vector design, the biology of the host response, immunology and toxicology and self assembly at the nanoscale. Clinical applications include the therapies of medical technology and regenerative medicine in all clinical disciplines, and diagnostic systems that reply on innovative contrast and sensing agents. The journal is relevant to areas such as cancer diagnosis and therapy, implantable devices, drug delivery systems, gene vectors, bionanotechnology and tissue engineering.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


