Impact of humic acid on iron (oxyhydr)oxide transport in the presence of phosphate in saturated porous media
IF 3.5
3区 环境科学与生态学
Q2 ENVIRONMENTAL SCIENCES
引用次数: 0
Abstract
The subsurface flow of particular phosphate (P) has been recently regarded as a vital P transport path. Humic acid (HA) and P usually coexist in the natural environment and show a strong affinity to iron (Fe) (oxyhydr)oxide. The impact of P and HA on Fe (oxyhydr)oxide stability and transport is critical for evaluating the vertical transport of particular P and biogeochemical processes of Fe and P. This study investigated the effect of inorganic (IP) and organic (OP) phosphate on the stability and transport of ferrihydrite and goethite with HA through stability tests and column experiments. The adsorption of IP or OP on Fe (oxyhydr)oxide enhanced the stability and transport of Fe (oxyhydr)oxide, and OP showed a stronger enhancement than IP due to its stronger binding capacity and more negative surface. Compared with ferrihydrite, goethite had fewer adsorption sites for IP or OP and showed strong stability and transport at low IP (50 μM) or OP (10 μM) concentration. HA decreased IP or OP adsorption on Fe (oxyhydr)oxide through competition adsorption and electrostatic repulsion. The formed ternary phosphate-Fe (oxyhydr)oxide-HA complex showed a more negative surface and strong stability and transport. Our findings provide direct insights into the distinct role of IP and OP on Fe (oxyhydr)oxide stability and transport in the presence of HA, which provides essential information for evaluating the transport of particular Fe (oxyhydr)oxide-facilitated P in soils and subsurface environments rich in iron, phosphate, and dissolved carbon.
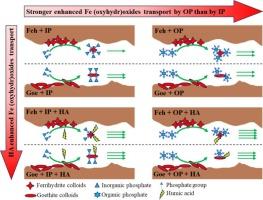
饱和多孔介质中磷酸盐存在时腐植酸对铁(氧氢)氧化物迁移的影响
最近,特定磷酸盐(P)的地下流动被视为重要的 P 运输途径。腐殖酸(HA)和磷通常共存于自然环境中,并与氧化铁(Fe)有很强的亲和力。本研究通过稳定性测试和柱实验研究了无机磷酸盐(IP)和有机磷酸盐(OP)对含有 HA 的铁水石和鹅铁矿的稳定性和迁移的影响。IP或OP对氧化铁的吸附增强了氧化铁的稳定性和迁移性,其中OP因其更强的结合能力和更负的表面而比IP表现出更强的增强作用。与无水亚铁相比,网纹石对 IP 或 OP 的吸附位点较少,在低浓度 IP(50 μM)或 OP(10 μM)时表现出较强的稳定性和迁移性。HA 通过竞争吸附和静电排斥作用减少了 IP 或 OP 在氧化铁上的吸附。形成的三元磷酸盐-铁(氧氢)氧化物-HA 复合物显示出更负的表面,具有很强的稳定性和迁移性。我们的研究结果直接揭示了 IP 和 OP 在 HA 存在时对氧化铁稳定性和迁移的不同作用,这为评估特定氧化铁促进的磷在富含铁、磷酸盐和溶解碳的土壤和地下环境中的迁移提供了重要信息。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of contaminant hydrology
环境科学-地球科学综合
CiteScore
6.80
自引率
2.80%
发文量
129
审稿时长
68 days
期刊介绍:
The Journal of Contaminant Hydrology is an international journal publishing scientific articles pertaining to the contamination of subsurface water resources. Emphasis is placed on investigations of the physical, chemical, and biological processes influencing the behavior and fate of organic and inorganic contaminants in the unsaturated (vadose) and saturated (groundwater) zones, as well as at groundwater-surface water interfaces. The ecological impacts of contaminants transported both from and to aquifers are of interest. Articles on contamination of surface water only, without a link to groundwater, are out of the scope. Broad latitude is allowed in identifying contaminants of interest, and include legacy and emerging pollutants, nutrients, nanoparticles, pathogenic microorganisms (e.g., bacteria, viruses, protozoa), microplastics, and various constituents associated with energy production (e.g., methane, carbon dioxide, hydrogen sulfide).
The journal''s scope embraces a wide range of topics including: experimental investigations of contaminant sorption, diffusion, transformation, volatilization and transport in the surface and subsurface; characterization of soil and aquifer properties only as they influence contaminant behavior; development and testing of mathematical models of contaminant behaviour; innovative techniques for restoration of contaminated sites; development of new tools or techniques for monitoring the extent of soil and groundwater contamination; transformation of contaminants in the hyporheic zone; effects of contaminants traversing the hyporheic zone on surface water and groundwater ecosystems; subsurface carbon sequestration and/or turnover; and migration of fluids associated with energy production into groundwater.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


