Highly enhanced electrocatalytic OER with facile electrodeposition of MIL–53(Fe)/NiAl–LDH/NF and NiAl–LDH/MIL–53(Fe)/NF
IF 4.7
3区 工程技术
Q2 ELECTROCHEMISTRY
引用次数: 0
Abstract
This research investigates a new approach to improve the electrocatalytic rate of the Oxygen Evolution Reaction (OER), a key step in water electrolysis. The study focuses on two promising materials: MIL–53(Fe) and NiAl–LDH. MIL–53(Fe) offers several advantages: high catalytic activity, large surface area, and good chemical stability. NiAl–LDH is attractive due to its layered structure, tolerance to a wide range of pH levels, scalability, and cost-effectiveness. However, their limitations like low conductivity and restricted accessibility of active sites hinder their performance in water splitting applications. To address these limitations, novel composite thin films were created using a technique called layer–by–layer (LBL) electrophoretic deposition. These films, built on nickel foam (NF) substrates, included two configurations: MIL–53(Fe)/NiAl–LDH/NF and NiAl–LDH/MIL–53(Fe)/NF. The MIL-53(Fe)/NiAl-LDH/NF composite exhibited remarkable OER activity in alkaline electrolytes, requiring overpotentials of only 200, 270, and 370 mV to reach current densities of 20, 50, and 100 mA cm−2, respectively. The Tafel slope of 54.86 mVdec−1 suggests rapid reaction kinetics. Additionally, it demonstrated excellent long-term stability, lasting for at least 20 h. The success of the MIL–53(Fe)/NiAl–LDH/NF composite can be attributed to the LBL technique. This method creates a composite with a larger surface area, significantly improving OER efficiency. In contrast, the MIL–53(Fe)/NiAl–LDH/NF configuration had the opposite effect. The NF pores became blocked by the MIL–53(Fe) layer, reducing the overall surface area, hindering electron transfer, and thereby limiting oxygen production. The LBL deposition method used in this study proves its effectiveness in designing efficient electrocatalysts. This opens up possibilities for creating other multicomponent materials for energy applications. The findings provide valuable insights for future research on these promising composite materials, potentially leading to the development of cost-effective and high-performance catalysts for various electrochemical applications.
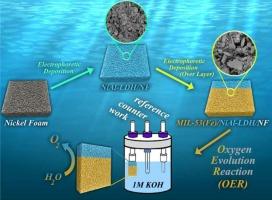
利用 MIL-53(Fe)/NiAl-LDH/NF 和 NiAl-LDH/MIL-53(Fe)/NF 的简易电沉积高度增强电催化 OER
这项研究探讨了一种提高氧进化反应(OER)电催化率的新方法,氧进化反应是水电解过程中的一个关键步骤。研究重点是两种有前途的材料:MIL-53(Fe) 和 NiAl-LDH。MIL-53(Fe) 具有几个优点:催化活性高、表面积大、化学稳定性好。NiAl-LDH 因其层状结构、对多种 pH 值的耐受性、可扩展性和成本效益而具有吸引力。然而,它们的局限性,如低电导率和活性位点访问受限,阻碍了它们在水分离应用中的性能。为了解决这些局限性,我们采用一种称为逐层(LBL)电泳沉积的技术制作了新型复合薄膜。这些薄膜建立在泡沫镍(NF)基底上,包括两种结构:MIL-53(Fe)/NiAl-LDH/NF和NiAl-LDH/MIL-53(Fe)/NF。在碱性电解质中,MIL-53(Fe)/NiAl-LDH/NF 复合材料表现出显著的 OER 活性,只需要 200、270 和 370 mV 的过电位就能分别达到 20、50 和 100 mA cm-2 的电流密度。54.86 mVdec-1 的塔菲尔斜率表明反应动力学速度很快。MIL-53(Fe)/NiAl-LDH/NF 复合材料的成功归功于 LBL 技术。这种方法能使复合材料具有更大的表面积,从而显著提高 OER 效率。相比之下,MIL-53(Fe)/NiAl-LDH/NF 配置则产生了相反的效果。NF 孔隙被 MIL-53(Fe)层阻塞,减少了整体表面积,阻碍了电子传递,从而限制了氧气的产生。本研究中使用的 LBL 沉积方法证明了其在设计高效电催化剂方面的有效性。这为创造其他多组分能源应用材料提供了可能性。这些发现为今后研究这些前景广阔的复合材料提供了宝贵的启示,有可能为各种电化学应用开发出具有成本效益的高性能催化剂。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Electrochemistry Communications
工程技术-电化学
CiteScore
8.50
自引率
3.70%
发文量
160
审稿时长
1.2 months
期刊介绍:
Electrochemistry Communications is an open access journal providing fast dissemination of short communications, full communications and mini reviews covering the whole field of electrochemistry which merit urgent publication. Short communications are limited to a maximum of 20,000 characters (including spaces) while full communications and mini reviews are limited to 25,000 characters (including spaces). Supplementary information is permitted for full communications and mini reviews but not for short communications. We aim to be the fastest journal in electrochemistry for these types of papers.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


