Biomimetic design of elastomers with improved mechanical properties by integrating hydrogen bonds with covalent crosslinking bonds
IF 4.5
3区 工程技术
Q1 CHEMISTRY, APPLIED
引用次数: 0
Abstract
It is an important issue for rubber to realize reinforcement and expand application gallery in material science and engineering. In the present work, we initiated a bioinspired design to introduce sacrificial hydrogen bonds by post-modifying sulfur crosslinked carboxyl nitrile rubber with pendent urazole through triazolinedione “click” chemistry. The post-modification of the crosslinked rubber can not only avoid adverse effect of the grafted urazoles on rubber vulcanization, but also enable to evaluate the effect of sacrificial hydrogen bonds on the rubber based on a consistent covalent network. Under external stress, the hydrogen bonds between the urazoles can function as sacrificial bonds that would rupture prior to covalent bonds, during which enormous energy is dissipated and meanwhile facilitates the rubber chain orientation. In light of the unique energy dissipating mechanism, the modulus, ultimate stress, and toughness of the hydrogen bonds and sulfur bonds dual-crosslinked rubber material are improved simultaneously without sacrificing the stretchability, and these properties can be easily manipulated by varying the grafting ratio of urazoles.
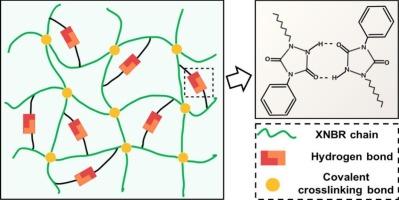
通过整合氢键和共价交联键,仿生设计具有更好机械性能的弹性体
在材料科学与工程领域,如何实现橡胶的补强并拓展其应用领域是一个重要课题。在本研究中,我们利用生物启发设计,通过三唑二酮 "点击 "化学将硫交联的羧基丁腈橡胶与下垂的脲唑进行后改性,从而引入牺牲氢键。对交联橡胶进行后改性不仅能避免接枝脲唑对橡胶硫化的不利影响,还能在一致的共价网络基础上评估牺牲氢键对橡胶的影响。在外部应力作用下,脲佐唑之间的氢键可作为牺牲键发挥作用,在共价键断裂之前就会断裂,在断裂过程中会耗散巨大的能量,同时促进橡胶链的定向。鉴于这种独特的能量耗散机制,氢键和硫键双交联橡胶材料的模量、极限应力和韧性在不牺牲拉伸性的情况下同时得到了改善,而且这些性能可以通过改变乌拉唑的接枝比例轻松实现。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Reactive & Functional Polymers
工程技术-高分子科学
CiteScore
8.90
自引率
5.90%
发文量
259
审稿时长
27 days
期刊介绍:
Reactive & Functional Polymers provides a forum to disseminate original ideas, concepts and developments in the science and technology of polymers with functional groups, which impart specific chemical reactivity or physical, chemical, structural, biological, and pharmacological functionality. The scope covers organic polymers, acting for instance as reagents, catalysts, templates, ion-exchangers, selective sorbents, chelating or antimicrobial agents, drug carriers, sensors, membranes, and hydrogels. This also includes reactive cross-linkable prepolymers and high-performance thermosetting polymers, natural or degradable polymers, conducting polymers, and porous polymers.
Original research articles must contain thorough molecular and material characterization data on synthesis of the above polymers in combination with their applications. Applications include but are not limited to catalysis, water or effluent treatment, separations and recovery, electronics and information storage, energy conversion, encapsulation, or adhesion.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


