Synthesis of azaflavanones and alpha-ylidene azaflavanones
IF 2.1
3区 化学
Q2 CHEMISTRY, ORGANIC
引用次数: 0
Abstract
Azaflavanones are synthetic aza analogues of natural flavanones that have received attention in medicine. More recently, some alpha-ylidene azaflavanones have emerged as promising bioactive compounds. In the last decades, several approaches to synthesizing azaflavanones and alpha-ylidene azaflavanones have been described. These include intramolecular cyclizations of 2′-aminochalcones and analogues, coupling of 2-aminoacetophenones with aromatic aldehydes, metal-catalyzed coupling reactions of ortho-iodoanilines and the condensations of N-protected 2-aminoacetophenones and N-protected 2′-aminochalcones with aromatic aldehydes. This review discusses the different approaches in the racemic synthesis of azaflavanones and alpha-ylidene azaflavanones.
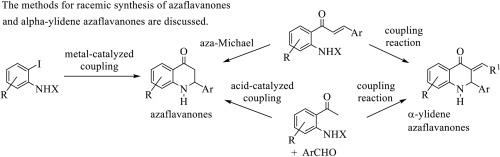
氮杂黄烷酮和α-亚基氮杂黄烷酮的合成
氮杂黄烷酮是天然黄烷酮的人工合成氮杂类似物,在医学领域备受关注。最近,一些α-亚基氮杂黄烷酮作为有前景的生物活性化合物出现。在过去的几十年中,人们已经描述了几种合成氮杂黄烷酮和α-亚基氮杂黄烷酮的方法。这些方法包括 2′-氨基查耳酮及其类似物的分子内环化反应、2-氨基苯乙酮与芳香醛的偶联反应、金属催化的邻碘苯胺偶联反应以及 N 保护的 2-氨基苯乙酮和 N 保护的 2′-氨基查耳酮与芳香醛的缩合反应。本综述讨论了外消旋合成氮杂黄烷酮和α-亚基氮杂黄烷酮的不同方法。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Tetrahedron
化学-有机化学
CiteScore
3.90
自引率
4.80%
发文量
439
审稿时长
34 days
期刊介绍:
Tetrahedron publishes full accounts of research having outstanding significance in the broad field of organic chemistry and its related disciplines, such as organic materials and bio-organic chemistry.
Regular papers in Tetrahedron are expected to represent detailed accounts of an original study having substantially greater scope and details than that found in a communication, as published in Tetrahedron Letters.
Tetrahedron also publishes thematic collections of papers as special issues and ''Reports'', commissioned in-depth reviews providing a comprehensive overview of a research area.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


