Insecticide binding mode analysis and biological effects of acetylcholinesterase target-site resistance mutations in Spodoptera frugiperda
IF 4.2
1区 农林科学
Q2 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
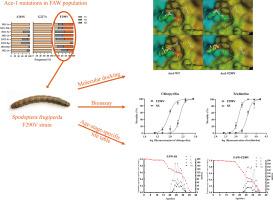
It is urgent to solve insecticide resistance issues for fall armyworm (FAW), Spodoptera frugiperda. Some acetylcholinesterase-1 (Ace-1) mutations (A201S, G227A and F290V) have been identified as a cause of FAW resistance to organophosphates (OPs) and carbamates insecticides (CXs). However, the structural biological mechanisms on the relationship between the Ace-1 mutations and resistance to OPs and CXs still remain elusive. In this study, the A201S and F290V mutaions were found in eight fields populations of FAW except the G227A. Molecular docking revealed that the four Ace-1 proteins (Ace1-WT, Ace1-A201S, Ace1-G227A and Ace1-F290V) had the same binding modes and the same binding energies with acetylcholine (Ach), trichlorfon, chlorpyrifos, methomyl, carbaryl and chlorpyrifos oxide. The structural biological analysis revealed that the A201S mutations can enhance enzyme catalytic efficiency by introducing the hydroxyl group (-OH) from serine which performed the same function as the main-chain -NH and enhanced the interaction with the carboxy oxygen of acetylcholine (Ach), and the F290V mutation can effectively improve FAW resistance to insecticides by increasing the likelihood of Ach to enter the enzyme's active center for phenylalanine replaced by smaller valine under insecticide inhibition conditions. The bioassays and age-stage-specific life table analysis of FAW-SS and FAW-F290V populations revealed that F290V mutation effectively contributed to FAW resistance with a low fitness cost. This study provides a theoretical basis for future pest resistance management.
蛙翅蝶乙酰胆碱酯酶靶位抗性突变的杀虫剂结合模式分析及生物效应
解决秋绵虫(FAW)(Spodoptera frugiperda)的杀虫剂抗性问题迫在眉睫。一些乙酰胆碱酯酶-1(Ace-1)突变(A201S、G227A 和 F290V)已被确认为导致秋绵虫对有机磷类杀虫剂(OPs)和氨基甲酸酯类杀虫剂(CXs)产生抗性的原因。然而,Ace-1 基因突变与 OPs 和 CXs 抗性之间的结构生物学机制仍未确定。在本研究中,除 G227A 突变外,A201S 和 F290V 突变均出现在 8 个 FAW 田间种群中。分子对接显示,四种 Ace-1 蛋白(Ace1-WT、Ace1-A201S、Ace1-G227A 和 Ace1-F290V)与乙酰胆碱(Ach)、敌百虫、毒死蜱、灭多威、克百威和氧化毒死蜱的结合模式和结合能相同。结构生物学分析表明,A201S突变可通过从丝氨酸中引入羟基(-OH)提高酶的催化效率,该羟基与主链上的-NH具有相同的功能,并增强了与乙酰胆碱(Ach)羧基氧的相互作用;F290V突变可在杀虫剂抑制条件下,通过用较小的缬氨酸取代苯丙氨酸增加Ach进入酶活性中心的可能性,从而有效提高一汽大众对杀虫剂的抗性。对FAW-SS和FAW-F290V种群的生物测定和特定年龄阶段的生命表分析表明,F290V突变以较低的适应性成本有效地提高了FAW的抗性。该研究为未来害虫抗性管理提供了理论依据。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
7.00
自引率
8.50%
发文量
238
审稿时长
4.2 months
期刊介绍:
Pesticide Biochemistry and Physiology publishes original scientific articles pertaining to the mode of action of plant protection agents such as insecticides, fungicides, herbicides, and similar compounds, including nonlethal pest control agents, biosynthesis of pheromones, hormones, and plant resistance agents. Manuscripts may include a biochemical, physiological, or molecular study for an understanding of comparative toxicology or selective toxicity of both target and nontarget organisms. Particular interest will be given to studies on the molecular biology of pest control, toxicology, and pesticide resistance.
Research Areas Emphasized Include the Biochemistry and Physiology of:
• Comparative toxicity
• Mode of action
• Pathophysiology
• Plant growth regulators
• Resistance
• Other effects of pesticides on both parasites and hosts.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


