Substantial oxygen loss and chemical expansion in lithium-rich layered oxides at moderate delithiation
IF 38.5
1区 材料科学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
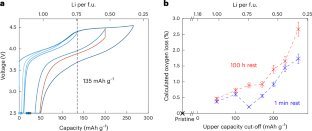
Delithiation of layered oxide electrodes triggers irreversible oxygen loss, one of the primary degradation modes in lithium-ion batteries. However, the delithiation-dependent mechanisms of oxygen loss remain poorly understood. Here we investigate the oxygen non-stoichiometry in Li1.18–xNi0.21Mn0.53Co0.08O2–δ electrodes as a function of Li content by using cycling protocols with long open-circuit voltage steps at varying states of charge. Surprisingly, we observe substantial oxygen loss even at moderate delithiation, corresponding to 2.5, 4.0 and 7.6 ml O2 per gram of Li1.18–xNi0.21Mn0.53Co0.08O2–δ after resting at upper capacity cut-offs of 135, 200 and 265 mAh g−1 for 100 h. Our observations suggest an intrinsic oxygen instability consistent with predictions of high oxygen activity at intermediate potentials versus Li/Li+. In addition, we observe a large chemical expansion coefficient with respect to oxygen non-stoichiometry, which is about three times greater than those of classical oxygen-deficient materials such as fluorite and perovskite oxides. Our work challenges the conventional wisdom that deep delithiation is a necessary condition for oxygen loss in layered oxide electrodes and highlights the importance of calendar ageing for investigating oxygen stability. Li-rich oxides were found to slowly release considerable quantities of oxygen at varying states of charge. These observations point to the intrinsic instability of oxygen in partially charged oxides and highlight the need for degradation studies.

富锂层状氧化物在中度脱锂时的大量氧损耗和化学膨胀
层状氧化物电极的脱ithiation 会引发不可逆的氧损耗,这是锂离子电池的主要降解模式之一。然而,人们对依赖于脱锂的氧损耗机制仍然知之甚少。在此,我们通过在不同充电状态下使用长开路电压阶跃的循环协议,研究了 Li1.18-xNi0.21Mn0.53Co0.08O2-δ 电极中的氧非化学计量与锂含量的关系。令人惊讶的是,我们观察到即使在适度脱硫的情况下也有大量氧气流失,在 135、200 和 265 mAh g-1 的上限容量截止点静置 100 小时后,每克 Li1.18-xNi0.21Mn0.53Co0.08O2-δ中的氧气含量分别为 2.5、4.0 和 7.6 毫升。此外,我们还观察到与氧非化学计量有关的巨大化学膨胀系数,该系数约为经典缺氧材料(如萤石和过氧化物氧化物)的三倍。我们的研究挑战了传统观点,即深度脱硫是层状氧化物电极中氧损失的必要条件,并强调了日历老化对研究氧稳定性的重要性。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature Materials
工程技术-材料科学:综合
CiteScore
62.20
自引率
0.70%
发文量
221
审稿时长
3.2 months
期刊介绍:
Nature Materials is a monthly multi-disciplinary journal aimed at bringing together cutting-edge research across the entire spectrum of materials science and engineering. It covers all applied and fundamental aspects of the synthesis/processing, structure/composition, properties, and performance of materials. The journal recognizes that materials research has an increasing impact on classical disciplines such as physics, chemistry, and biology.
Additionally, Nature Materials provides a forum for the development of a common identity among materials scientists and encourages interdisciplinary collaboration. It takes an integrated and balanced approach to all areas of materials research, fostering the exchange of ideas between scientists involved in different disciplines.
Nature Materials is an invaluable resource for scientists in academia and industry who are active in discovering and developing materials and materials-related concepts. It offers engaging and informative papers of exceptional significance and quality, with the aim of influencing the development of society in the future.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


