Neuropeptide signalling orchestrates T cell differentiation
IF 50.5
1区 综合性期刊
Q1 MULTIDISCIPLINARY SCIENCES
引用次数: 0
Abstract
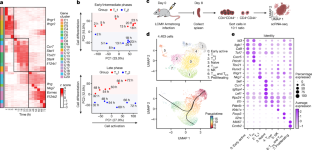
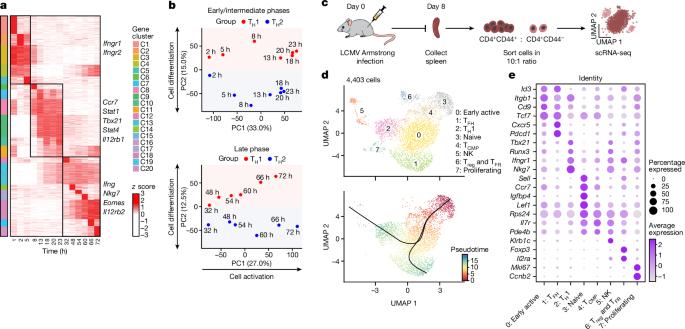
The balance between T helper type 1 (TH1) cells and other TH cells is critical for antiviral and anti-tumour responses1–3, but how this balance is achieved remains poorly understood. Here we dissected the dynamic regulation of TH1 cell differentiation during in vitro polarization, and during in vivo differentiation after acute viral infection. We identified regulators modulating T helper cell differentiation using a unique TH1–TH2 cell dichotomous culture system and systematically validated their regulatory functions through multiple in vitro and in vivo CRISPR screens. We found that RAMP3, a component of the receptor for the neuropeptide CGRP (calcitonin gene-related peptide), has a cell-intrinsic role in TH1 cell fate determination. Extracellular CGRP signalling through the receptor RAMP3–CALCRL restricted the differentiation of TH2 cells, but promoted TH1 cell differentiation through the activation of downstream cAMP response element-binding protein (CREB) and activating transcription factor 3 (ATF3). ATF3 promoted TH1 cell differentiation by inducing the expression of Stat1, a key regulator of TH1 cell differentiation. After viral infection, an interaction between CGRP produced by neurons and RAMP3 expressed on T cells enhanced the anti-viral IFNγ-producing TH1 and CD8+ T cell response, and timely control of acute viral infection. Our research identifies a neuroimmune circuit in which neurons participate in T cell fate determination by producing the neuropeptide CGRP during acute viral infection, which acts on RAMP3-expressing T cells to induce an effective anti-viral TH1 cell response. RAMP3, a component of the receptor for the neuropeptide CGRP, has a cell-intrinsic role in T helper type 1 cell fate determination.
神经肽信号协调 T 细胞分化
1 型 T 辅助细胞(TH1)和其他 TH 细胞之间的平衡对抗病毒和抗肿瘤反应至关重要1,2,3,但人们对如何实现这种平衡仍然知之甚少。在这里,我们剖析了体外极化过程中以及急性病毒感染后体内分化过程中 TH1 细胞分化的动态调控。我们利用独特的 TH1-TH2 细胞二分培养系统鉴定了调节 T 辅助细胞分化的调控因子,并通过多个体外和体内 CRISPR 筛选系统地验证了它们的调控功能。我们发现,作为神经肽 CGRP(降钙素基因相关肽)受体的一个组成部分,RAMP3 在 TH1 细胞命运决定中具有细胞内在的作用。通过受体 RAMP3-CALCRL 发出的细胞外 CGRP 信号限制了 TH2 细胞的分化,但通过激活下游的 cAMP 反应元件结合蛋白(CREB)和激活转录因子 3(ATF3)促进了 TH1 细胞的分化。ATF3通过诱导TH1细胞分化的关键调节因子Stat1的表达来促进TH1细胞的分化。病毒感染后,神经元产生的 CGRP 与 T 细胞表达的 RAMP3 之间的相互作用增强了 TH1 和 CD8+ T 细胞产生的抗病毒 IFNγ 反应,并及时控制了急性病毒感染。我们的研究发现了一个神经免疫回路,在这个回路中,神经元在急性病毒感染期间通过产生神经肽CGRP参与T细胞命运的决定,而CGRP则作用于表达RAMP3的T细胞,诱导有效的抗病毒TH1细胞反应。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature
综合性期刊-综合性期刊
CiteScore
90.00
自引率
1.20%
发文量
3652
审稿时长
3 months
期刊介绍:
Nature is a prestigious international journal that publishes peer-reviewed research in various scientific and technological fields. The selection of articles is based on criteria such as originality, importance, interdisciplinary relevance, timeliness, accessibility, elegance, and surprising conclusions. In addition to showcasing significant scientific advances, Nature delivers rapid, authoritative, insightful news, and interpretation of current and upcoming trends impacting science, scientists, and the broader public. The journal serves a dual purpose: firstly, to promptly share noteworthy scientific advances and foster discussions among scientists, and secondly, to ensure the swift dissemination of scientific results globally, emphasizing their significance for knowledge, culture, and daily life.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


