Methylarginine targeting chimeras for lysosomal degradation of intracellular proteins
IF 12.9
1区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
A paradigm shift in drug development is the discovery of small molecules that harness the ubiquitin-proteasomal pathway to eliminate pathogenic proteins. Here we provide a modality for targeted protein degradation in lysosomes. We exploit an endogenous lysosomal pathway whereby protein arginine methyltransferases (PRMTs) initiate substrate degradation via arginine methylation. We developed a heterobifunctional small molecule, methylarginine targeting chimera (MrTAC), that recruits PRMT1 to a target protein for induced degradation in lysosomes. MrTAC compounds degraded substrates across cell lines, timescales and doses. MrTAC degradation required target protein methylation for subsequent lysosomal delivery via microautophagy. A library of MrTAC molecules exemplified the generality of MrTAC to degrade known targets and neo-substrates—glycogen synthase kinase 3β, MYC, bromodomain-containing protein 4 and histone deacetylase 6. MrTAC selectively degraded target proteins and drove biological loss-of-function phenotypes in survival, transcription and proliferation. Collectively, MrTAC demonstrates the utility of endogenous lysosomal proteolysis in the generation of a new class of small molecule degraders. Development of a targeted protein degradation platform, methylarginine targeting chimera (MrTAC), enables arginine methylation-driven degradation of intracellular proteins in lysosomes.
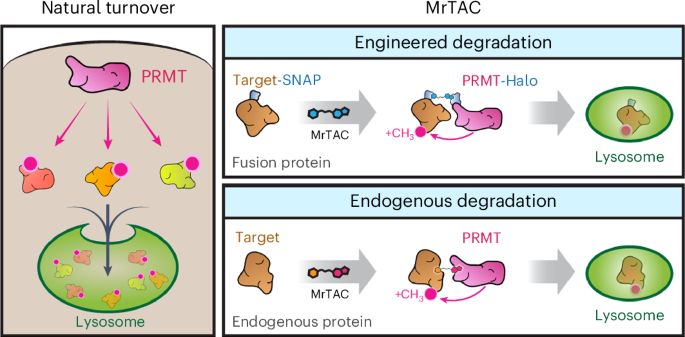
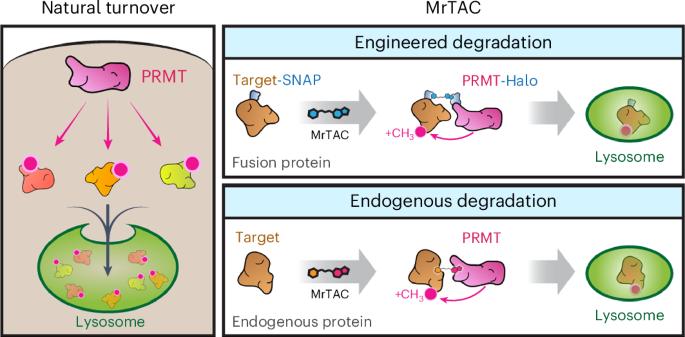
用于细胞内蛋白质溶酶体降解的甲基精氨酸靶向嵌合体
药物开发的一个范式转变是发现利用泛素-蛋白酶体途径消除致病蛋白质的小分子。在这里,我们提供了一种在溶酶体中靶向降解蛋白质的方法。我们利用了一种内源性溶酶体途径,即蛋白质精氨酸甲基转移酶(PRMTs)通过精氨酸甲基化启动底物降解。我们开发了一种杂多功能小分子--甲基精氨酸靶向嵌合体(Methylarginine targeting chimera,MrTAC),它能将 PRMT1 募集到靶蛋白上,诱导其在溶酶体中降解。MrTAC化合物降解的底物跨越细胞系、时间尺度和剂量。MrTAC降解需要靶蛋白甲基化,以便随后通过微自噬作用进入溶酶体。MrTAC分子库体现了MrTAC降解已知靶标和新底物--糖原合酶激酶3β、MYC、含溴结构域蛋白4和组蛋白去乙酰化酶6--的通用性。MrTAC 可选择性地降解靶蛋白,并导致生存、转录和增殖方面的生物功能缺失表型。总之,MrTAC 证明了内源性溶酶体蛋白水解在生成一类新型小分子降解剂中的作用。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature chemical biology
生物-生化与分子生物学
CiteScore
23.90
自引率
1.40%
发文量
238
审稿时长
12 months
期刊介绍:
Nature Chemical Biology stands as an esteemed international monthly journal, offering a prominent platform for the chemical biology community to showcase top-tier original research and commentary. Operating at the crossroads of chemistry, biology, and related disciplines, chemical biology utilizes scientific ideas and approaches to comprehend and manipulate biological systems with molecular precision.
The journal embraces contributions from the growing community of chemical biologists, encompassing insights from chemists applying principles and tools to biological inquiries and biologists striving to comprehend and control molecular-level biological processes. We prioritize studies unveiling significant conceptual or practical advancements in areas where chemistry and biology intersect, emphasizing basic research, especially those reporting novel chemical or biological tools and offering profound molecular-level insights into underlying biological mechanisms.
Nature Chemical Biology also welcomes manuscripts describing applied molecular studies at the chemistry-biology interface due to the broad utility of chemical biology approaches in manipulating or engineering biological systems. Irrespective of scientific focus, we actively seek submissions that creatively blend chemistry and biology, particularly those providing substantial conceptual or methodological breakthroughs with the potential to open innovative research avenues. The journal maintains a robust and impartial review process, emphasizing thorough chemical and biological characterization.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


