Li2CO3/LiF-Rich solid electrolyte interface stabilized lithium metal anodes for durable Li-CO2 batteries
IF 20.2
1区 材料科学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
Lithium carbon dioxide (Li-CO2) batteries are considered a promising next-generation energy storage device due to their high theoretical energy density and potential carbon neutralization. Despite numerous iterative advancements in cathode catalysts for Li-CO2 batteries, the cycling stability still to be hindered by the growth of lithium dendrites during cycling, primarily due to uneven deposition and the side reaction of sufficient CO2 with the Li metal anode. In this work, bisalt electrolyte (BE) consisting of LiPF6 and LiTFSI is used as a localized anode surface stabilizer to achieve durable Li-CO2 batteries. The introduction of PF6- promotes the decomposition and reduction of TFSI−, leading to the formation of LiF-rich inorganic SEI (Li2CO3/LiF-rich) with enhanced Li+ affinity and good electronic insulating properties. This effectively inhibits lithium dendrite formation while also insulating CO2 and electrolytes from contacting the lithium anode. Consequently, the Li symmetric battery incorporating the novel BE exhibits a long cycling life of 912 h (∼3.8 times of the cell with a single-salt electrolyte (SE)). The BE based Li-CO2 battery achieves an ultra-long cyclelife of 2720 h (∼2.6 times of SE battery) and outstanding rate capability. In addition, the assembled belt-shaped Li-CO2 batteries could stably power a digital watch for 1267 h.
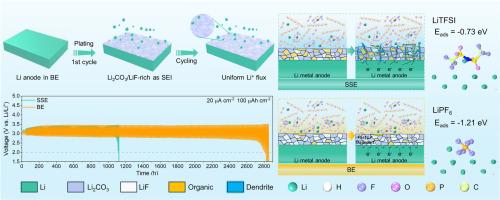
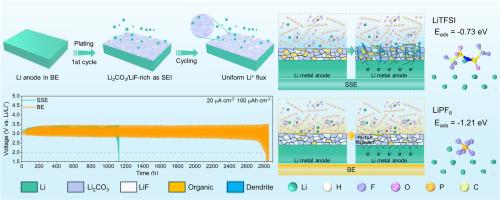
用于耐用锂-CO2 电池的 Li2CO3/LiF 富固体电解质界面稳定锂金属阳极
二氧化碳锂电池(Li-CO2)因其理论能量密度高、碳中和潜力大而被认为是一种前景广阔的下一代储能设备。尽管锂-CO2 电池的正极催化剂迭有进步,但其循环稳定性仍然受到循环过程中锂枝晶生长的阻碍,这主要是由于沉积不均匀以及充足的二氧化碳与锂金属阳极发生副反应造成的。在这项研究中,由 LiPF6 和 LiTFSI 组成的双盐电解质(BE)被用作局部阳极表面稳定剂,以实现耐用的锂-CO2 电池。PF6- 的引入促进了 TFSI- 的分解和还原,从而形成了富含 LiF 的无机 SEI(Li2CO3/LiF-rich),具有更强的 Li+ 亲和力和良好的电子绝缘性能。这不仅能有效抑制锂枝晶的形成,还能隔绝二氧化碳和电解质与锂阳极的接触。因此,采用新型 BE 的锂对称电池的循环寿命长达 912 小时(是采用单盐电解质(SE)电池的 3.8 倍)。基于 BE 的二氧化碳锂电池实现了 2720 小时的超长循环寿命(是 SE 电池的 2.6 倍)和出色的速率能力。此外,组装后的腰带型锂-CO2 电池可为数字手表稳定供电 1267 小时。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Energy Storage Materials
Materials Science-General Materials Science
CiteScore
33.00
自引率
5.90%
发文量
652
审稿时长
27 days
期刊介绍:
Energy Storage Materials is a global interdisciplinary journal dedicated to sharing scientific and technological advancements in materials and devices for advanced energy storage and related energy conversion, such as in metal-O2 batteries. The journal features comprehensive research articles, including full papers and short communications, as well as authoritative feature articles and reviews by leading experts in the field.
Energy Storage Materials covers a wide range of topics, including the synthesis, fabrication, structure, properties, performance, and technological applications of energy storage materials. Additionally, the journal explores strategies, policies, and developments in the field of energy storage materials and devices for sustainable energy.
Published papers are selected based on their scientific and technological significance, their ability to provide valuable new knowledge, and their relevance to the international research community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


