New horizons in our understanding of precursor multiple myeloma and early interception
IF 72.5
1区 医学
Q1 ONCOLOGY
引用次数: 0
Abstract
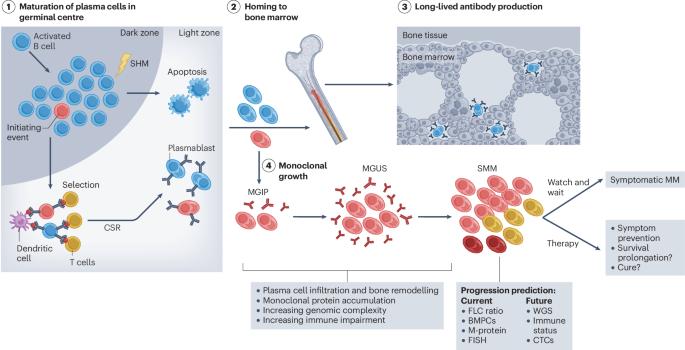
Multiple myeloma is an incurable plasma cell malignancy that evolves over decades through the selection and malignant transformation of monoclonal plasma cells. The evolution from precursor states to symptomatic disease is characterized by an increasing complexity of genomic alterations within the plasma cells and a remodelling of the microenvironment towards an immunosuppressive state. Notably, in patients with advanced disease, similar mechanisms of tumour escape and immune dysfunction mediate resistance to modern T cell-based therapies, such as T cell-engaging bispecific antibodies and chimeric antigen receptor (CAR)-T cells. Thus, an increasing number of clinical trials are assessing the efficiency and safety of these therapies in individuals with newly diagnosed multiple myeloma and high-risk smoldering multiple myeloma. In this Review, we summarize the current knowledge about tumour intrinsic and extrinsic processes underlying progression from precursor states to symptomatic myeloma and discuss the rationale for early interception including the use of T cell-redirecting therapies. Multiple myeloma is a plasma cell malignancy that is currently incurable. Cordas dos Santos et al. describe how multiple myeloma arises from precursor states and how T cell-redirecting therapies might be used to intercept disease progression at these earlier stages to improve patient outcomes.
我们对多发性骨髓瘤前兆和早期阻断的认识新视野
多发性骨髓瘤是一种无法治愈的浆细胞恶性肿瘤,通过单克隆浆细胞的选择和恶性转化,历经数十年演变而成。从前驱状态到有症状疾病的演变过程中,浆细胞内的基因组改变日益复杂,微环境也朝着免疫抑制状态重塑。值得注意的是,在晚期疾病患者中,类似的肿瘤逃逸和免疫功能失调机制介导了对基于 T 细胞的现代疗法的抵抗,如 T 细胞参与的双特异性抗体和嵌合抗原受体(CAR)-T 细胞。因此,越来越多的临床试验正在评估这些疗法在新诊断的多发性骨髓瘤和高风险的烟雾型多发性骨髓瘤患者中的有效性和安全性。在这篇综述中,我们总结了目前关于肿瘤从前驱状态发展到有症状骨髓瘤的内在和外在过程的知识,并讨论了早期阻断包括使用T细胞导向疗法的理由。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature Reviews Cancer
医学-肿瘤学
CiteScore
111.90
自引率
0.40%
发文量
97
审稿时长
6-12 weeks
期刊介绍:
Nature Reviews Cancer, a part of the Nature Reviews portfolio of journals, aims to be the premier source of reviews and commentaries for the scientific communities it serves. The correct abbreviation for abstracting and indexing purposes is Nat. Rev. Cancer. The international standard serial numbers (ISSN) for Nature Reviews Cancer are 1474-175X (print) and 1474-1768 (online). Unlike other journals, Nature Reviews Cancer does not have an external editorial board. Instead, all editorial decisions are made by a team of full-time professional editors who are PhD-level scientists. The journal publishes Research Highlights, Comments, Reviews, and Perspectives relevant to cancer researchers, ensuring that the articles reach the widest possible audience due to their broad scope.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


