Anion-cation coupled electrolyte additive enhancing the performance of lithium-oxygen batteries
IF 13.3
1区 工程技术
Q1 ENGINEERING, CHEMICAL
引用次数: 0
Abstract
The rational design of electrolyte via introducing additive is an efficient strategy to improve the electrochemical performance of lithium-oxygen batteries (LOBs). In this work, coupling LiCl and Sn(TFSI)2 as the anion-cation electrolyte additive achieved the high-performance of LOBs by promoting the oxygen electrode reactions, regulating the Li+ solvation structure, and inducing the growth of the SEI protective layer. Specifically, Cl− with strong coordination affinity to Li+ could weaken the binding between Li+ and TEGDME solvent molecules, leading to the formation of an anion-dominant Li+ solvation structure, which facilitates the diffusion of Li+ in the electrolyte bulk and electrolyte/electrode interface. On the other hand, Sn2+ can serve as a redox mediator to catalyze ORR on the cathode and participate in the growth of smooth and dense SEI protective layer on Li metal anode surface, thus effectively promoting the oxygen electrode reaction kinetics and suppressing the corrosion of Li anode. As a consequence, the LOBs with coupled LiCl and Sn(TFSI)2 additive exhibited a ultra-high discharge capacity of 21517.1 mA h g−1 and an excellent cycling life of 334 cycles. This work provides a deep understanding for designing efficient electrolyte with additive for LOBs.
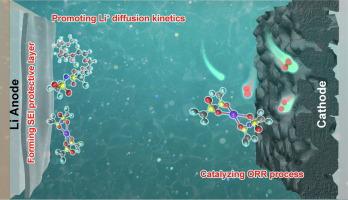
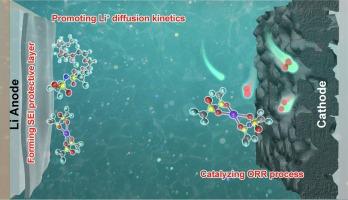
提高锂氧电池性能的阴阳离子耦合电解质添加剂
通过引入添加剂合理设计电解质是提高锂氧电池(LOBs)电化学性能的有效策略。在这项工作中,将 LiCl 和 Sn(TFSI)2 作为阴阳离子电解质添加剂,通过促进氧电极反应、调节 Li+ 溶解结构和诱导 SEI 保护层生长,实现了锂氧电池的高性能。具体来说,与 Li+ 配位亲和力强的 Cl- 可削弱 Li+ 与 TEGDME 溶剂分子之间的结合力,从而形成阴离子主导的 Li+ 溶解结构,促进 Li+ 在电解质体和电解质/电极界面的扩散。另一方面,Sn2+ 可作为氧化还原介质催化阴极上的 ORR,并参与锂金属阳极表面光滑致密的 SEI 保护层的生长,从而有效促进氧电极反应动力学,抑制锂阳极的腐蚀。因此,添加了氯化锂和 Sn(TFSI)2 添加剂的 LOB 具有 21517.1 mA h g-1 的超高放电容量和 334 次循环的优异循环寿命。这项工作为设计带有添加剂的高效 LOB 电解质提供了深刻的理解。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Chemical Engineering Journal
工程技术-工程:化工
CiteScore
21.70
自引率
9.30%
发文量
6781
审稿时长
2.4 months
期刊介绍:
The Chemical Engineering Journal is an international research journal that invites contributions of original and novel fundamental research. It aims to provide an international platform for presenting original fundamental research, interpretative reviews, and discussions on new developments in chemical engineering. The journal welcomes papers that describe novel theory and its practical application, as well as those that demonstrate the transfer of techniques from other disciplines. It also welcomes reports on carefully conducted experimental work that is soundly interpreted. The main focus of the journal is on original and rigorous research results that have broad significance. The Catalysis section within the Chemical Engineering Journal focuses specifically on Experimental and Theoretical studies in the fields of heterogeneous catalysis, molecular catalysis, and biocatalysis. These studies have industrial impact on various sectors such as chemicals, energy, materials, foods, healthcare, and environmental protection.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


