Placebo Rates in Crohn’s Disease Randomized Clinical Trials: An Individual Patient Data Meta-Analysis
IF 25.7
1区 医学
Q1 GASTROENTEROLOGY & HEPATOLOGY
引用次数: 0
Abstract
Background & Aims
Understanding placebo rates is critical for efficient clinical trial design. We assessed placebo rates and associated factors using individual patient data from Crohn’s disease trials.
Methods
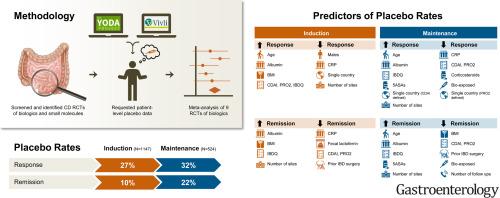
We conducted a meta-analysis of phase 2/3 placebo-controlled trials evaluating advanced therapies in moderate to severe Crohn’s disease (2010–2021). Deidentified individual patient data were obtained through Vivli Inc and the Yale University Open Data Access Project. Primary outcomes were clinical response and remission. Pooled placebo rates and 95% confidence intervals (CIs) were estimated using 1- and 2-stage meta-analytic approaches. Regression analyses identified patient-level factors associated with placebo rates.
Results
Analysis of individual patient data from 8 induction (n = 1147) and 4 maintenance (n = 524) trials showed overall placebo clinical response and remission rates for induction were 27% (95% CI, 23%–32%) and 10% (95% CI, 8%–14%), respectively, and 32% (95% CI, 23%–42%) and 22% (95% CI, 14%–33%) for maintenance, respectively. Among biologic (bio)-naïve patients, placebo response and remission rates during induction were 29% (95% CI, 24%–35%) and 11% (95% CI, 8%–15%) respectively, and 26% (95% CI, 20%–33%) and 10% (95% CI, 8%–14%) for biologic (bio)-exposed patients, respectively. During maintenance, biologic-naïve response and remission rates were 41% (95% CI, 34%–48%) and 32% (95% CI, 24%–40%), respectively, and 29% (95% CI, 24%–34%) and 16% (95% CI, 13%–21%) for bio-exposed, respectively. Higher baseline C-reactive protein concentration predicted lower placebo rates, whereas higher baseline albumin levels and body mass index increased the odds of placebo outcomes. Increased baseline Crohn’s Disease Activity Index and 2-item patient-reported outcome scores predicted higher response rates in induction, lower response rates in maintenance, and lower remission rates in induction and maintenance.
Conclusions
Patient- and trial-level characteristics influence placebo rates in Crohn’s disease trials. Careful implementation of eligibility criteria, outcome definitions, and patient stratification may reduce placebo rates.

克罗恩病随机临床试验中的安慰剂比例:患者个体数据荟萃分析
背景& 目的了解安慰剂使用率对于高效的临床试验设计至关重要。我们利用克罗恩病(CD)试验中的患者个体数据(IPD)评估了安慰剂率及相关因素。方法我们对评估中重度CD晚期疗法的2/3期安慰剂对照试验(2010-2021年)进行了荟萃分析。通过 Vivli 公司和耶鲁大学开放数据访问项目获得了去标识化 IPD。主要结果为临床反应和缓解。采用一阶段和两阶段荟萃分析方法估算了汇总安慰剂率和 95% 置信区间 (CI)。结果利用 8 项诱导试验(n=1147)和 4 项维持试验(n=524)的 IPD,诱导试验的总体安慰剂临床应答率和缓解率分别为 27% (95%CI=23-32%) 和 10% (95%CI=8-14%),维持试验的总体安慰剂临床应答率和缓解率分别为 32% (95%CI=23-42%) 和 22% (95%CI=14-33%)。在生物无效患者中,诱导期安慰剂应答率和缓解率分别为29%(95%CI=24-35%)和11%(95%CI=8-15%),生物暴露期分别为26%(95%CI=20-33%)和10%(95%CI=8-14%)。在维持治疗期间,生物无效反应率和缓解率分别为41%(95%CI=34-48%)和32%(95%CI=24-40%),生物暴露率分别为29%(95%CI=24-34%)和16%(95%CI=13-21%)。较高的基线C反应蛋白浓度预示着较低的安慰剂比例,而较高的基线白蛋白水平和体重指数则增加了安慰剂结果的几率。较高的基线克罗恩病活动指数和2项患者报告结果评分预示着较高的诱导应答率、较低的维持应答率以及较低的诱导和维持缓解率。谨慎执行资格标准、结果定义和患者分层可降低安慰剂使用率。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Gastroenterology
医学-胃肠肝病学
CiteScore
45.60
自引率
2.40%
发文量
4366
审稿时长
26 days
期刊介绍:
Gastroenterology is the most prominent journal in the field of gastrointestinal disease. It is the flagship journal of the American Gastroenterological Association and delivers authoritative coverage of clinical, translational, and basic studies of all aspects of the digestive system, including the liver and pancreas, as well as nutrition.
Some regular features of Gastroenterology include original research studies by leading authorities, comprehensive reviews and perspectives on important topics in adult and pediatric gastroenterology and hepatology. The journal also includes features such as editorials, correspondence, and commentaries, as well as special sections like "Mentoring, Education and Training Corner," "Diversity, Equity and Inclusion in GI," "Gastro Digest," "Gastro Curbside Consult," and "Gastro Grand Rounds."
Gastroenterology also provides digital media materials such as videos and "GI Rapid Reel" animations. It is abstracted and indexed in various databases including Scopus, Biological Abstracts, Current Contents, Embase, Nutrition Abstracts, Chemical Abstracts, Current Awareness in Biological Sciences, PubMed/Medline, and the Science Citation Index.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


