Application of aluminosilicate residue-based zeolite from lithium extraction in water treatment
IF 4.8
3区 材料科学
Q1 CHEMISTRY, APPLIED
引用次数: 0
Abstract
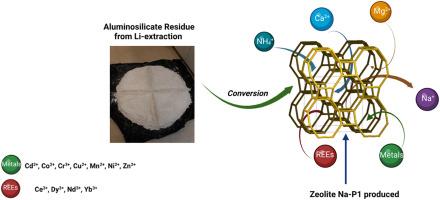
In a previous study, the Na-P1 type zeolites were synthesized from aluminosilicate residues using an efficient and cost-effective process, exhibiting an excellent adsorption capacity for Ca2+ in comparison to commercial zeolites 13X and A. Building upon this, the current study evaluates their performance for the adsorption of various elements, including Ca2+, Mg2+, and NH4+. The objective was to evaluate the performance of the Na-P1 type zeolites for the adsorption of various elements, including Ca2+, Mg2+, and rare earth elements, with a particular emphasis on the adsorption kinetics and water hardness removal in comparison to commercial zeolite A. The results demonstrated that the Na-P1 zeolite exhibited a satisfactory sorption capacity for Ca2+ and NH4+ ions (66 mg/g), while displaying a relatively lower effectiveness for the sorption of Mg2+ ions (5.6 mg/g). The Langmuir model is particularly well suited to the sorption of Ca2+, while the Freundlich model is more appropriate for Mg2+. Both models demonstrated satisfactory representation of NH₄ ⁺ sorption. Moreover, the pseudo-second-order kinetic model provides an excellent description of the Ca2⁺ and Mg2⁺ sorption processes, while both models effectively describe the NH₄⁺ adsorption kinetics. Additionally, Na-P1 zeolite was observed to effectively reduce water hardness from 322 to 63 mg CaCO₃/L at temperatures of 10, 20, and 38 °C, and to 18 mg/L at 58 °C. These findings suggest that Na-P1 zeolite has promising potential for applications as a water softening agent. Regarding metals and rare earths, the Na-P1 zeolite demonstrated noteworthy sorption efficiencies for Cd2+ (138 mg/g), Ce3+ (209 mg/g), Cr3+ (56.2 mg/g), and Cu2+ (60.5 mg/g). However, it demonstrated lower sorption efficiencies for Co2+, Mn2+, Ni2+ and Dy3+ (below 16 mg/g). The findings illustrate that Na-P1 zeolites are effective for the adsorption of diverse elements, offering a promising avenue for the sustainable transformation of industrial waste into valuable materials for environmental applications.
在水处理中应用提取锂的铝硅酸盐残渣基沸石
在之前的一项研究中,利用高效、经济的工艺从硅酸铝残渣中合成了 Na-P1 型沸石,与商用沸石 13X 和 A 相比,Na-P1 型沸石对 Ca2+ 具有出色的吸附能力。在此基础上,本研究评估了它们对 Ca2+、Mg2+ 和 NH4+ 等各种元素的吸附性能。结果表明,Na-P1 型沸石对 Ca2+ 和 NH4+ 离子(66 毫克/克)的吸附能力令人满意,而对 Mg2+ 离子(5.6 毫克/克)的吸附效果相对较低。Langmuir 模型特别适合 Ca2+ 的吸附,而 Freundlich 模型则更适合 Mg2+。这两种模型都能令人满意地反映 NH⁺ 的吸附情况。此外,伪二阶动力学模型很好地描述了 Ca2⁺和 Mg2⁺的吸附过程,而两种模型都有效地描述了 NH₄⁺ 的吸附动力学。此外,在温度为 10、20 和 38 °C 时,Na-P1 沸石可有效地将水硬度从 322 毫克 CaCO₃/L 降至 63 毫克 CaCO₃/L,在 58 °C 时降至 18 毫克/L。这些研究结果表明,Na-P1 沸石具有作为水软化剂的应用潜力。在金属和稀土方面,Na-P1 沸石对 Cd2+(138 毫克/克)、Ce3+(209 毫克/克)、Cr3+(56.2 毫克/克)和 Cu2+(60.5 毫克/克)具有显著的吸附效率。不过,它对 Co2+、Mn2+、Ni2+ 和 Dy3+(低于 16 毫克/克)的吸附效率较低。研究结果表明,Na-P1 沸石能有效吸附多种元素,为可持续地将工业废物转化为有价值的环境应用材料提供了一条前景广阔的途径。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Microporous and Mesoporous Materials
化学-材料科学:综合
CiteScore
10.70
自引率
5.80%
发文量
649
审稿时长
26 days
期刊介绍:
Microporous and Mesoporous Materials covers novel and significant aspects of porous solids classified as either microporous (pore size up to 2 nm) or mesoporous (pore size 2 to 50 nm). The porosity should have a specific impact on the material properties or application. Typical examples are zeolites and zeolite-like materials, pillared materials, clathrasils and clathrates, carbon molecular sieves, ordered mesoporous materials, organic/inorganic porous hybrid materials, or porous metal oxides. Both natural and synthetic porous materials are within the scope of the journal.
Topics which are particularly of interest include:
All aspects of natural microporous and mesoporous solids
The synthesis of crystalline or amorphous porous materials
The physico-chemical characterization of microporous and mesoporous solids, especially spectroscopic and microscopic
The modification of microporous and mesoporous solids, for example by ion exchange or solid-state reactions
All topics related to diffusion of mobile species in the pores of microporous and mesoporous materials
Adsorption (and other separation techniques) using microporous or mesoporous adsorbents
Catalysis by microporous and mesoporous materials
Host/guest interactions
Theoretical chemistry and modelling of host/guest interactions
All topics related to the application of microporous and mesoporous materials in industrial catalysis, separation technology, environmental protection, electrochemistry, membranes, sensors, optical devices, etc.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


