The optical and thermoelectric properties of layer structured Ba2XS4 (X = Zr, Hf) for energy harvesting applications
IF 4.3
3区 材料科学
Q2 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
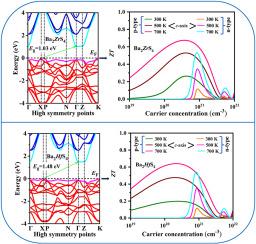
The main objective of this research is to provide a comprehensive insight into the optical and thermoelectric properties of layer structured Ba2XS4(X = Zr, Hf) for energy harvesting applications using Density Functional Theory (DFT) and semiclassical Boltzmann transport theory. There is a good match between the computed lattice parameters and the available experimental data. Both compounds are thermodynamically and mechanically stable and they are soft, ductile, machinable, and elastically anisotropic. The indirect band gaps are found to be 1.03 eV for Ba2ZrS4 and 1.48 eV for Ba2HfS4. Both compounds possess a mixture of ionic and covalent bonding confirmed by charge density distribution and Mulliken bond population analysis. The maximum absorption is in the ultraviolet regions ( of light spectra. The total thermal conductivity increases with temperature due to increasing trend of electronic thermal conductivity. The total thermal conductivity at 700 K along c-axis is 4.6 (6.1 W/mK) for Ba2ZrS4 (Ba2HfS4). For p-type Ba2ZrS4 (Ba2HfS4), power factor (PF) is about 7 (5.7) mW/mK2, whereas for n-type it is about 4 (3.9) mW/mK2 at 700 K along c-axis. The power factors of the studied compounds are much higher than those of the reported GeTe and SnSe which would create great interest for further study. The predicted ZT values at 700 K for p-type Ba2ZrS4 and Ba2HfS4 are 0.7 and 0.6, respectively. These values may further be improved through reduction of thermal conductivity and tuning ductility employing known suitable strategies such as alloying and nano-structuring. Finally, Ba2ZrS4 and Ba2HfS4 can be considered new eco-friendly alternatives to previously studied toxic lead-based thermoelectric materials. Their unique advantages of high thermodynamic stability, non-toxic nature and high performance make them strong candidate for sustainable energy solutions.
用于能量收集应用的层状结构 Ba2XS4(X = Zr、Hf)的光学和热电特性
本研究的主要目的是利用密度泛函理论(DFT)和半经典波尔兹曼输运理论,对用于能量收集应用的层状结构 Ba2XS4(X = Zr、Hf)的光学和热电特性进行全面深入的研究。计算得出的晶格参数与现有实验数据非常吻合。这两种化合物都具有热力学和机械稳定性,而且柔软、韧性好、可加工,并具有弹性各向异性。研究发现,Ba2ZrS4 和 Ba2HfS4 的间接带隙分别为 1.03 eV 和 1.48 eV。电荷密度分布和 Mulliken 键群分析证实,这两种化合物都具有离子键和共价键。最大吸收位于光光谱的紫外区(∼13.6eV)。由于电子热导率呈上升趋势,总热导率随温度升高而增加。对于 Ba2ZrS4 (Ba2HfS4),700 K 时沿 c 轴的总热导率为 4.6 (6.1 W/mK)。对于 p 型 Ba2ZrS4 (Ba2HfS4),功率因数 (PF) 约为 7 (5.7) mW/mK2,而对于 n 型 Ba2ZrS4 (Ba2HfS4),在 700 K 时沿 c 轴的功率因数约为 4 (3.9) mW/mK2。所研究化合物的功率因数远高于已报道的 GeTe 和 SnSe,这引起了进一步研究的极大兴趣。p 型 Ba2ZrS4 和 Ba2HfS4 在 700 K 时的预测 ZT 值分别为 0.7 和 0.6。通过采用已知的合适策略(如合金化和纳米结构)降低热导率和调整延展性,这些值可能会进一步提高。最后,Ba2ZrS4 和 Ba2HfS4 可以被认为是新的生态友好型材料,可替代以前研究过的有毒铅基热电材料。它们具有热力学稳定性高、无毒和高性能等独特优势,是可持续能源解决方案的有力候选材料。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
7.80
自引率
2.50%
发文量
605
审稿时长
40 days
期刊介绍:
The Journal of Physics and Chemistry of Solids is a well-established international medium for publication of archival research in condensed matter and materials sciences. Areas of interest broadly include experimental and theoretical research on electronic, magnetic, spectroscopic and structural properties as well as the statistical mechanics and thermodynamics of materials. The focus is on gaining physical and chemical insight into the properties and potential applications of condensed matter systems.
Within the broad scope of the journal, beyond regular contributions, the editors have identified submissions in the following areas of physics and chemistry of solids to be of special current interest to the journal:
Low-dimensional systems
Exotic states of quantum electron matter including topological phases
Energy conversion and storage
Interfaces, nanoparticles and catalysts.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


