Microneedles integrated with crystallinity control for poorly water-soluble drugs: Enhanced bioavailability and innovative controlled release system
IF 7.6
2区 材料科学
Q1 MATERIALS SCIENCE, MULTIDISCIPLINARY
引用次数: 0
Abstract
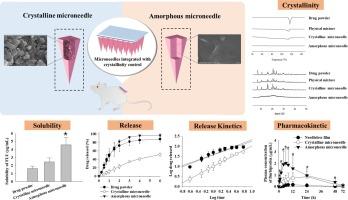
The purpose of this study was to develop innovative microneedles with drug crystallinity control for the fast or sustained release of poorly water-soluble drugs. Povidone was determined as a suitable polymer following hydrophilic polymer testing using solubilization screening technique. Microneedles were fabricated by altering the drug-to-polymer weight ratios. Their mechanical properties, crystallinity, solubility, release, skin permeability and transdermal pharmacokinetics in rats were assessed. The optimal crystalline and amorphous microneedles were composed of drug/polymer at weight ratios of 1:0.03 and 1:2.5, respectively. They showed excellent insertion in rat skin with a puncture rate above 80%. Compared to drug powder or solution, they increased drug solubility, release and skin permeability. Crystalline microneedles gave sustained release and plasma concentration profiles, while amorphous microneedles provided a fast profile. Amorphous microneedles offered significantly faster Tmax and two-fold higher area under the concentration–time curve (AUC), indicating better transdermal bioavailability. In the safety test, microneedle-treated rat skin was recovered to normal within three days without any irritations. Thus, the drug crystallinity could play a significant role in the release of microneedles, suggesting their potential as a transdermal drug delivery system for controlling the release of poorly water-soluble drugs and improving their transdermal bioavailability.
微针与结晶度控制相结合,适用于水溶性差的药物:提高生物利用率和创新型控释系统
本研究旨在开发具有药物结晶度控制功能的创新型微针,用于快速或持续释放水溶性较差的药物。在使用增溶筛选技术对亲水性聚合物进行测试后,确定聚维酮是一种合适的聚合物。通过改变药物与聚合物的重量比,制造出了微针。对微针的机械性能、结晶度、溶解度、释放、皮肤渗透性和大鼠的透皮药代动力学进行了评估。最佳结晶微针和无定形微针的药物/聚合物重量比分别为 1:0.03 和 1:2.5。这些微针在大鼠皮肤上的插入效果极佳,穿刺率超过 80%。与药物粉末或溶液相比,它们提高了药物的溶解度、释放度和皮肤渗透性。晶体微针具有持续释放和血浆浓度分布,而非晶体微针则具有快速分布。无定形微针的Tmax明显更快,浓度-时间曲线下面积(AUC)高出两倍,表明其透皮生物利用率更高。在安全性测试中,经过微针处理的大鼠皮肤在三天内恢复正常,没有出现任何刺激症状。因此,药物结晶度在微针的释放过程中起着重要作用,这表明微针有可能成为一种透皮给药系统,用于控制水溶性差的药物的释放并提高其透皮生物利用度。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
Materials & Design
Engineering-Mechanical Engineering
CiteScore
14.30
自引率
7.10%
发文量
1028
审稿时长
85 days
期刊介绍:
Materials and Design is a multi-disciplinary journal that publishes original research reports, review articles, and express communications. The journal focuses on studying the structure and properties of inorganic and organic materials, advancements in synthesis, processing, characterization, and testing, the design of materials and engineering systems, and their applications in technology. It aims to bring together various aspects of materials science, engineering, physics, and chemistry.
The journal explores themes ranging from materials to design and aims to reveal the connections between natural and artificial materials, as well as experiment and modeling. Manuscripts submitted to Materials and Design should contain elements of discovery and surprise, as they often contribute new insights into the architecture and function of matter.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


