Longitudinal Evaluation of Individuals With Severe Alpha-1 Antitrypsin Deficiency (Pi∗ZZ Genotype)
IF 25.7
1区 医学
Q1 GASTROENTEROLOGY & HEPATOLOGY
引用次数: 0
Abstract
Background & Aims
Homozygous Pi∗Z mutation in alpha-1 antitrypsin (Pi∗ZZ genotype) predisposes to pulmonary loss-of-function and hepatic gain-of-function injury. To facilitate selection into clinical trials typically targeting only 1 organ, we systematically evaluated an international, multicenter, longitudinal, Pi∗ZZ cohort to uncover natural disease course and surrogates for future liver- and lung-related endpoints.
Methods
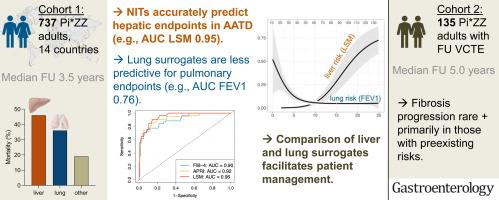
Cohort 1 recruited 737 Pi∗ZZ individuals from 25 different centers without known liver comorbidities who received a baseline clinical and laboratory assessment as well as liver stiffness measurement (LSM). A follow-up interview was performed after at least 6 months. Cohort 2 consisted of 135 Pi∗ZZ subjects without significant liver fibrosis, who received a standardized baseline and follow-up examination at least 2 years later, both including LSM.
Results
During 2634 patient-years of follow-up, 39 individuals died, with liver and lung being responsible for 46% and 36% of deaths, respectively. Forty-one Pi∗ZZ subjects who developed a hepatic endpoint presented with significantly higher baseline liver fibrosis surrogates, that is, LSM (24 vs 5 kPa, P < .001) and aspartate aminotransferase–to-platelet ratio index (1.1 vs 0.3 units, P < .001). Liver-related endpoints within 5 years were most accurately predicted by LSM (area under the curve 0.95) followed by aspartate aminotransferase–to-platelet ratio index (0.92). Baseline lung parameters displayed only a moderate predictive utility for lung-related endpoints within 5 years (forced expiratory volume in the first second area under the curve 0.76). Fibrosis progression in those with no/mild fibrosis at baseline was rare and primarily seen in those with preexisting risk factors.
Conclusions
Noninvasive liver fibrosis surrogates accurately stratify liver-related risks in Pi∗ZZ individuals. Our findings have direct implications for routine care and future clinical trials of Pi∗ZZ patients.

对严重α-1 抗胰蛋白酶缺乏症(Pi*ZZ 基因型)患者进行纵向评估
背景和目的α-1抗胰蛋白酶(Pi*ZZ 基因型)的杂合子 Pi*Z 突变易导致肺功能缺失和肝功能增益损伤。为了便于选择参加通常只针对一个器官的临床试验,我们对一个国际性、多中心、纵向的 Pi*ZZ 队列进行了系统评估,以揭示自然病程以及未来肝脏和肺脏相关终点的替代指标。方法队列 1 从 25 个不同中心招募了 737 名 Pi*ZZ 患者,他们没有已知的肝脏合并症,接受了基线临床和实验室评估以及肝脏硬度测量(LSM)。至少六个月后进行了一次随访。队列2由135名无明显肝纤维化的Pi*ZZ受试者组成,他们接受了标准化的基线检查和至少两年后的随访检查,均包括肝硬度测量。41名出现肝脏终点的Pi*ZZ受试者的基线肝纤维化替代指标,即LSM(24对5千帕,p<.001)和谷草转氨酶与血小板比值指数(APRI,1.1对0.3单位,p<.001)显著较高。LSM 对五年内肝脏相关终点的预测最为准确(曲线下面积 [AUC] 0.95),其次是 APRI(0.92)。肺部基线参数对五年内肺部相关终点的预测作用一般(FEV1 AUC 0.76)。基线无纤维化/轻度纤维化的患者很少出现纤维化进展,主要见于已有风险因素的患者。结论非侵入性肝纤维化替代指标能准确地对 Pi*ZZ 患者的肝脏相关风险进行分层。我们的研究结果对Pi*ZZ患者的日常护理和未来临床试验具有直接意义。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Gastroenterology
医学-胃肠肝病学
CiteScore
45.60
自引率
2.40%
发文量
4366
审稿时长
26 days
期刊介绍:
Gastroenterology is the most prominent journal in the field of gastrointestinal disease. It is the flagship journal of the American Gastroenterological Association and delivers authoritative coverage of clinical, translational, and basic studies of all aspects of the digestive system, including the liver and pancreas, as well as nutrition.
Some regular features of Gastroenterology include original research studies by leading authorities, comprehensive reviews and perspectives on important topics in adult and pediatric gastroenterology and hepatology. The journal also includes features such as editorials, correspondence, and commentaries, as well as special sections like "Mentoring, Education and Training Corner," "Diversity, Equity and Inclusion in GI," "Gastro Digest," "Gastro Curbside Consult," and "Gastro Grand Rounds."
Gastroenterology also provides digital media materials such as videos and "GI Rapid Reel" animations. It is abstracted and indexed in various databases including Scopus, Biological Abstracts, Current Contents, Embase, Nutrition Abstracts, Chemical Abstracts, Current Awareness in Biological Sciences, PubMed/Medline, and the Science Citation Index.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


